Homogeneous Mixture vs Heterogeneous Mixture – Types of Solutions
An unadulterated substance can be a component, or an intensify that are artificially homogenous in creation and can’t be isolated by any physical methods. A few instances of an unadulterated substance would be Iron Metal (Fe), Salt (NaCl) and so forth.
Most characteristic substances, and pretty much anything one could consider, is doubtlessly a blend. Air, water, soil, milk. Obviously, there are distinctive sorts of blends, yet comprehensively one could characterize every one of the things found normally in presence as a blend.
Things being what they are, what is a blend?
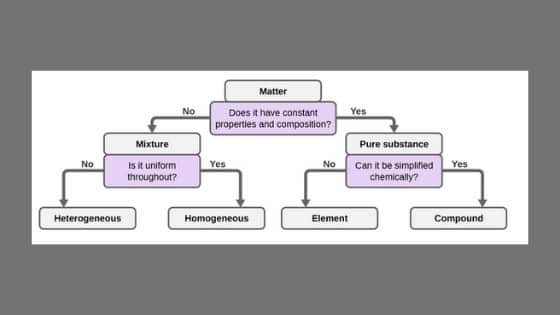
A blend is essentially a mix of at least two substances that are not synthetically joined together and don’t exist in settled extents to one another. A blend can be part into unadulterated substances – mixes or components.
A blend may have diverse physical properties; for instance, a blend of water and liquor bubbles over a scope of temperatures relying upon a great deal of elements.
MIXTURES
A blend can be physically isolated into unadulterated mixes or elements.
Most normally happening substances are blends. Indeed, even the most flawless of materials still contain different mixes as impurities.
Blends may display a changing arrangement of physical properties.
For instance, blend of liquor and water bubbles over a scope of temperatures.
PURE COMPOUNDS
An unadulterated compound has a steady piece with settled proportions of components.
Although it is physically difficult to disconnect unadulterated substances, a substance is said to be unadulterated if no contamination’s can be identified utilizing the best accessible scientific systems.
Physical properties, for example, breaking point or dissolving purpose of unadulterated substances are invariant.
For instance, unadulterated water bubbles at 100 degrees Celsius
Sorts of Mixtures
In science, blends are once in a while called homogeneous or heterogeneous. The contrast between them is the degree to which, and how consistently, their distinctive parts are combined.
For instance, on the off chance that you have container of nails and fastens front of you, you can see plainly that it is comprised of various parts, yet take a gander at a jug of milk, and all you see is a white fluid.
Homogenous Mixtures
Homogeneous blends have a similar uniform appearance and structure all through. These comprise of particles as little as iota’s or atoms; as such, too little to possibly be obvious. It’s difficult to choose parts of a homogeneous blend. For instance, a sugar arrangement or a blend of water and liquor are homogeneous in light of the fact that just vapid fluids can be seen.
Homogeneous blends just have one stage: gas, fluid or strong. Different homogenous blends are air, water and vodka.
Heterogeneous Mixtures
Heterogeneous blends are comprised of noticeably extraordinary substances or stages. A suspension is a kind of heterogeneous blend with huge particles, obvious.
For instance, a blend of sand and water is a suspension since you can see the sand particles in the water. In like manner, plate of mixed greens dressing made of oil and vinegar is a suspension since you can see two fluid layers. Different heterogeneous blends are mists in air, oat in milk, blood, nourishment, sand among others.
As a rule, it is conceivable to physically isolate parts of a heterogeneous blend, however not a homogeneous blend. For instance, you can expel oat from milk and pasta from sauce. On the off chance that you are uncertain about whether a blend is homogeneous or heterogeneous, consider its example estimate. Some heterogeneous blends can seem homogeneous from a separation, for example, sand on a shoreline. On the off chance that the creation of a blend seems uniform regardless of where you test it, is homogeneous; sand on a shoreline is heterogeneous in light of the fact that when you take a gander at it up intently, you can distinguish diverse kinds of particles, for example, sand, shells and natural issue.
HOMOGENEOUS MIXTURES
- The prefixes “homo”- demonstrate sameness. A homogeneous blend has a similar uniform appearance and structure all through. Numerous homogeneous blends are generally alluded to as solutions.Molecule estimate recognizes homogeneous arrangements from different heterogeneous blends.
- Arrangements have particles which are the span of iotas or atoms – too little to even think about being seen.Corn oil is homogeneous, White vinegar is homogeneous. A sugar arrangement is homogeneous since just a boring fluid is watched. Air without any mists is homogeneous.
- The prefixes: “hetero”- shows distinction.
A heterogeneous blend comprises of unmistakably extraordinary substances or stages. The three stages or conditions of issue are gas, fluid, and strong. - Realistic on the left of “Moving Raisins” indicates fluid, strong, and gas substances in a heterogeneous blend.
- In differentiate a suspension is a heterogeneous blend of bigger particles. These particles are obvious and will settle out on standing. Instances of suspensions are fine sand or sediment in water or tomato juice.
- For precedent, shoreline sand is heterogeneous since you can see diverse shaded particles. Oil and water are heterogeneous as two fluid layers are available, just as solids. Air with mists is heterogeneous, as the mists contain small beads of fluid water.
A colloid is a homogeneous arrangement with moderate molecule measure between an answer and a suspension. Colloid particles might be found in a light emission, for example, dust in air in a beam of daylight. Milk, mist, and jam are instances of colloids.
An answer is a blend of at least two substances in a solitary stage. No less than two substances must be blended so as to have an answer. The substance in the littlest sum and the one that breaks up or scatters is known as the Solute. The substance in the bigger sum is known as the Solvent. In most basic occurrences water is the dissolvable. The gases, fluids, or solids broke down in water are the solutes.
In the realistic, the blue jug is a homogeneous arrangement blend of water, KOH, glucose, oxygen gas broke down, and methylene blue – a marker.
Since arrangements are blends, their creations may fluctuate over a wide range. The fixations might be communicated utilizing an assortment of measures. The non-explicit terms focused and weaken are once in a while utilized. A concentrated arrangement has a generally extensive (however non-explicit) measure of solute broke up in a dissolvable. A weaken arrangement has a littler amount of solute broke down.
Types of Solutions
| Solute | Solvent | Examples |
| Less than 50% | More than 50% | |
| liquid | liquid | alcohol – water |
| solid | liquid | salt – water |
| gas | liquid | oxygen – water |
| gas | gas | air = oxygen – nitrogen |
| gas | solid | hydrogen – platinum |
| liquid | gas | water in air |
| solid | gas | smog |
| liquid | solid | mercury – another metal |
| solid | solid | alloy |
Division of Mixtures
Division of heterogenous blends is to a great extent physical and is very tedious. We make utilization of various properties of the material to proceed with their partitions.
For a compound response to be described further it is important to detach the parts from different materials. Different examinations like bio compound frameworks, natural investigation and pharmaceutical research requires solid detachment strategies.
Here are a few basic division methods:
Chromatography
Chromatography is a technique for isolating a blend by passing it in arrangement or as gasy) through a medium in which the parts move at various rates. Slim layer chromatography is a unique sort of chromatography utilized for isolating and distinguishing blends that are or can be shaded, particularly colours.
Distillation

Distillation is a technique to isolate blends included at least two unadulterated fluids or an answer. Distillation is a procedure of decontamination where the fluid blend is vaporized, dense and disconnected. In straight forward refining, a blend is warmed, and the most unstable segment vaporizes in any event temperature. The vapour goes through a cooled cylinder (a condenser), where it consolidates once more into its fluid state. The condensate that is gathered is called distillate.
In the Figure above, we see a few essential bits of hardware. There is a warmth source, a test tube with a one-gap plug joined to a glass elbow and elastic tubing. The elastic tubing is put into a gathering tube which is submerged in virus water. There are other progressively confused gatherings for distillation that can likewise be utilized, particularly to isolate blends, which are included unadulterated fluids with breaking points that are near each other.
Evaporation
Evaporation is a procedure used to isolate out homogenous blends where there is at least one broken up solids. This strategy drives off the fluid segments from the strong parts. The procedure commonly includes warming the blend until not any more fluid stays, prior to utilizing this strategy; the blend should just contain one fluid part, except if it isn’t essential to disengage the fluid segments. This is on the grounds that every fluid part will dissipate after some time. This strategy is appropriate to isolate a solvent strong from a fluid.
In numerous parts of the world, table salt is gotten from the evaporation of ocean water. The warmth for the procedure originates from the sun.
When the ocean water in these vanishing lakes has dissipated, the salt can be gathered.
Filtration
Filtration is a partition strategy used to isolate out unadulterated substances in blends involved particles some of which are sufficiently huge in size to be caught with a permeable material. Molecule size can change impressively, given the sort of blend. For example, stream water is a blend that contains normally happening natural life forms like microscopic organisms, infections, and protozoan’s. Some water channels can sift through microscopic organisms, the length of which is on the request of 1 micron. Different blends, similar to soil, have moderately huge molecule sizes, which can be separated through something like an espresso channel.
Fractional Distillation
Fractional distillation is utilized for the partition of a blend of at least two miscible fluids for which the distinction in breaking points is under 25K. The device for partial refining resembles that of straight forward refining; then again, actually a fractionating section is fitted in the middle of the refining cup and the condenser.
A basic fractionating segment is a cylinder pressed with glass dots. The globules give surface to the vapours to cool and gather over and over. At the point when vapours of a blend are gone through the fractionating segment, in light of the rehashed build-up and dissipation, the vapours of the fluid with the lower breaking point first go out of the fractionating segment, gather and are gathered in the collector flagon. The other fluid, with a somewhat higher breaking point, can be gathered in comparable style in another receiver vessel.
Centrifugation
Once in a while the strong particles in a fluid are exceptionally little and can go through a channel paper. For such particles, the filtration procedure can’t be utilized for partition. Such blends are isolated by centrifugation. In this way, centrifugation is the procedure of partition of insoluble materials from a fluid where ordinary filtration does not function admirably. The centrifugation depends on the size, shape, and thickness of the particles, consistency of the medium, and the speed of pivot. The standard is that the denser particles are compelled to the base and the lighter particles remain at the best when spun quickly.
The device utilized for centrifugation is known as a rotator. The axis comprises of a rotator tube holder called rotor. The rotor holds adjusted diffusive containers of equivalent measures of the strong fluid blend. On quick pivot of the rotor, the rotator tubes turn on a level plane and because of the divergent power, the denser insoluble particles separate from the fluid. At the point when the revolution stops, the strong particles end up at the base of the rotator tube with fluid at the best.