NEET Chemistry is the scoring paper in the medical entrance examination. Here, you will discover the NEET Chemistry MCQ Questions for all Concepts as per the latest syllabus. Practice more on a regular basis with these NEET Chemistry objective questions on air pollution and improve your subject knowledge & problem-solving skills along with time management. NEET Chemistry States of Matter Multiple Choice Questions make you feel confident in answering the question in the exam & increases your scores to high.
MCQs on States of Matter
1. A container with a pin-hole contains equal moles of H2(g) and O2(g). Find the fraction of oxygen gas escaped at the same time when one-fourth of hydrogen gas escapes
(a) 1/16
(b) 1/4
(c) 1/2
(d) 1/8
Answer
Answer: (a)
2. What are the conditions for gas like Carbon monoxide to obey the ideal gas laws?
(a) low temperature and low pressure
(b) low temperature and high pressure
(c) high temperature and low pressure
(d) high temperature and high pressure
Answer
Answer: (c)
3. If the temperature is doubled, the average velocity of a gaseous molecule increases by
(a) 4
(b) 1.4
(c) 2
(d) 2.8
Answer
Answer: (b)
4. Find the molecular mass of a gas that takes three times more time to effuse as compared to He with the same volume
(a) 9 u
(b) 64 u
(c) 27 u
(d) 36 u
Answer
Answer: (d)
5. At the same temperature, the average molar kinetic energy of N2 and CO is
(a) KE1 > KE2
(b) KE1 < KE2
(c) KE1 = KE2
(d) insufficient information given
Answer
Answer: (c)
6. Find the temperature at which the rate of effusion of N2 is 1.625 times to that of SO2 at 500℃
(a) 620℃
(b) 173℃
(c) 110℃
(d) 373℃
Answer
Answer: (a)
7. Find the change in the root mean square speed of the gas on raising the temperature from 27℃ to 927℃
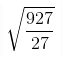
(a) becomes times
(b) gets doubled
(c) gets halved
(d) remains same
Answer
Answer: (b)
8. Find the relation between probable velocity, mean velocity and root mean square velocity
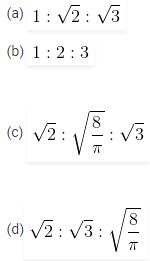
Answer
Answer: (c)
9. If 1.204 x 1021 molecules of H2SO4 are removed from 392 mg of H2SO4, find the moles of H2SO4 left.
(a) 4 x 10-3
(b) 1.5 x 10-3
(c) 1.2 x 10-3
(d) 2 x 10-3
Answer
Answer: (d)
10. Find the fraction of the total pressure exerted by hydrogen if it is mixed with ethane in an empty container at 25℃
(a) 15/16
(b) 1/16
(c) 1/2
(d) 1
Answer
Answer: (a)