Metals and Nonmetals – Types, Properties and Differences
Introduction:
Every object around us can be categorized into two types of elements: metals or non-metals. Your books, clothes, pencil, water bottle, bag, table, the door are all examples of non-metals. Therefore, it is important to know the properties of Metals and Non-Metals and how to distinguish between them.

The periodic table:
The periodic table comprises an arrangement of elements based on certain chemical properties that they exhibit. The metals are arranged on the left side and the non-metals on the right side of the periodic table. The rows of the table are called periods and columns are called groups. There is a total of 92 elements that are known to be found naturally, out of which 70 are metals and 22 are non-metals.
Metals:
In the above depiction of the periodic table, most of the elements are metals. There are various kinds of metals:
- alkaline earth metals,
- alkali metals,
- transition metals,
- actinides, and
- lanthanides

Metals which are placed on the left-hand side of the periodic table are separated from non-metals by a zigzag line that starts from Carbon (C) and runs down Phosphorus (P), Selenium (Se), Iodine (I), till Radon (Rn). Therefore, these chemical elements and everything on their right is non-metal and the row just to their left is known as semi-metals or metalloids. They have properties that are common to both metals and non-metals.

Physical Properties:
- Metals occur in the solid state. All metals are solid except with an exception for mercury which is in liquid state in its natural form.
- Metals are malleable in nature. They can be beaten into thin sheets. For example, elements such as aluminium, gold, and silver can be beaten into thin sheets for common usage purpose.
- Metals are ductile. This means that metals can be stretched into thin wires. We can make copper wires and aluminium wires. All metals are equally ductile. Only that some metals are more ductile than others for which they are used for day to day purposes.
- Metals conduct heat and electricity. It is by virtue of this property of metals that heat, and electricity can pass through them. Every metal is a good conductor of heat and electricity.
Note: Silver is the best conductor of heat and electricity, copper is also a good conductor. The worst conductor of heat is lead whereas Iron and mercury are poor conductors of electricity.
- Metals are shiny. It is due to this property of metals that they are lustrous, and they reflect the light incident on its surface. Also, metals can be polished, and this is one of the reasons why metals are used to make jewellery and desired by women and men alike.
- Metals are very strong and hard, exceptions being sodium and potassium. They can be cut with a knife.
- Metals are also known to be heavy.
- Metals are also sonorous. They produce a sound when they are rung or hit with any object.
- Metals have a high melting point and a high boiling point.
- Metals have high density.
- Metals in the form of objects are opaque and are never transparent or translucent.
Chemical Properties:
- Metals easily corrode very easily and fast.
- Metals lose electrons easily. Their outer shell has 1, 2 or 3 electrons.
- Most metals form metal oxides when they come in contact with the oxygen.
- Metals have low electro-negativities, they are electropositive elements.
- Metals are also good reducing agents.
Non-Metals:
The non-metal elements are those that do not possess the properties of metals. The number of non-metals on the periodic table is very less as compared to metals. Non-metals are positioned on the right side of the periodic table. Some examples of the non-metals are hydrogen, carbon, nitrogen, phosphorus, oxygen, sulphur, selenium, all the halogens, and the noble gases.


Physical Properties:
- Non-metals are brittle and break into pieces when beaten. Example: Sulphur and phosphorus.
- Non-metals are not ductile so, they cannot be made into thin wires.
- Non-metals are insulators or poor conductors of electricity and heat because they do not lose electrons to transmit the energy.
- At room temperature, they can be in the state of solids, liquids or gases.
- They are non-sonorous.
- They can be transparent.
Chemical Properties:
- Non-metals generally have somewhere around 4 to 8 electrons in the outer shell.
- Non-metals tend to gain or accept valence electrons.
- When they are exposed to oxygen, non-metals react with oxygen to form acidic oxides.
- Non-metals have high electro-negativity; they are electro-negative elements.
- Non-metal elements are good oxidizing agents.
- These elements do not react with water.
Comparison of physical properties of metals and non-metals:
| Property type | Metals | Non-metals |
| Physical State | Solid at room temperature. Exception being mercury and gallium. | Exist as solids and gases, exception being bromine. |
| Density | Highly dense | Low. |
| Melting and boiling points | High melting point and boiling point Exception being gallium and caesium. | Low melting point and boiling point. Exception being diamond and graphite. |
| Malleability and Ductility | malleable and ductile | not malleable or ductile. |
| Conductivity | Conducts heat and electricity | Poor/ bad conductors of heat and electricity exception being graphite. |
| Lustre | Shining lustre | They have no lustre except for iodine. |
| Sonorous sound | Sonorous. | Non-sonorous. |
| Hardness | Generally hard exception being Na, K | Generally soft except diamond |
Comparison of chemical properties of metals and non-metals:
| Reaction type | Metals | Non-metals |
| Reaction with H2O | Metals on reacting with water form metal oxides or metal hydroxides and release H2 gas. | Non-metals cannot give electrons to hydrogen in water to be released as H2gas. Non-metals have no reaction with water. |
| Reaction with O2 | Metals react with oxygen to form basic oxides.Zn and Al form amphoteric oxides which show the properties of both acidic and basic oxides.Mostly, metal oxides are insoluble in water. Some of them dissolve to form alkali. | Non-metals react with oxygen to form oxides.Non- metal oxides are soluble in water. They dissolve in water to form acids. |
| Reaction with acids | Metals react with acid to form salt and release hydrogen.When metals react with HNO3, H2 is not released. HNO3 is strong oxidizing agent. | No reaction with acids occurs to release H2 gas. Non-metals don’t lose electrons to give it to hydrogen ions of acids. |
| Reaction with salt solutions | When metals react with salt solution, more reactive metals displace less reactive metals from its salt solution. | Here, more reactive non-metals displace less reactive non-metals from its salt solution. |
| Reaction with chlorine | Metals react with chlorine to form metal chloride. It is an ionic bond.What we get is an ionic compound | Non-metals react with chlorine to form non-metal chloride. It forms a covalent bond. What we get is a co-valent compound. |
| Reaction with H2 | Only highly reactive metals react with hydrogen to form metal hydride. | Non-metals react with hydrogen to give hydrides. |
Table of reactivity series shows order in which the metals are arranged based on their comparative reactivity.
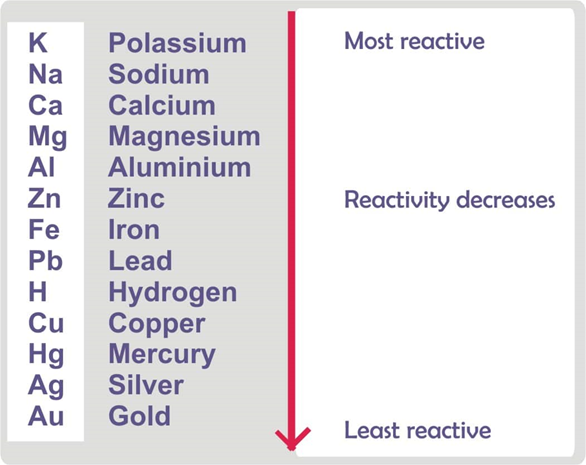
Steps involved in the extraction of metals from the ore:

Calcination and Roasting:
| Calcination | Roasting |
| In this process, ores are heated in the absence of oxygen where metal oxide is formed and CO2 releases.It is done for carbonate ores CaCO3 → CaO + CO2(g) | In this process, sulphur ore is heated in the presence of oxygen. Metal oxide is formed and SO2 gas releases.It is done for sulphide ores. ZnS+ 3O2 heat 3ZnO+ SO2 |
Questions
1. Take samples of Fe, Cu, Al, Mg and note the appearance of each sample.
2. Give an example of each:
i. Metal which is liquid at room temperature.
ii. Metal which can be easily cut with knife.
iii. A metal which is a good conductor of heat.
3. Explain the meaning of malleable and ductile.
4. What do you mean by displacement reaction?
5. Give one example of displacement reaction.
6. If you have ever seen a blacksmith beating an iron piece? What change did you find in the shape of these pieces on beating? Would you find a similar kind of change in wood log on beating?
7. Name two most malleable metals.
8. Prove the fact that metals are good conductors of electricity activity comes into equation.
9. List some physical properties of the metals.
10. Write some physical properties of non-metals.
11. What happens when sodium and water.
12. Why non – metals do not react with water?
13. Fill in the blanks:
i. Non- metal oxides are ……………… in water.
ii. Non-metals don’t lose electrons to give it to hydrogen ions of ……….
iii. In this calcination, ores are heated in the absence of oxygen where metal oxide is formed and ………………… releases.
iv. In roasting, In this perocess, metal oxide is obtained and ……………….. gas releases.
v. When metals react with salt solution, more reactive metals …………………. less reactive metals from its salt solution.
vi. Non-metals are …………… and break into pieces when beaten.
vii. Non-metals are not …………… so, they cannot be made into thin wires.
14. Give three reasons for the following:
(i) Why sulphur is a non-metal?
(ii) Why magnesium is a metal?
(iii) You are given three different samples of metals. Sodium, magnesium and copper. Write any two activities to arrange them in order of decreasing activity.