MCQ Questions for Class 6 Chemistry with Answers: Central Board of Secondary Education (CBSE) has declared a major change in the Class 6 exam pattern from 2020. Practicing & preparing each and every chapter covered in the CBSE Class 6 Science Syllabus is a necessary task to attempt the MCQs Section easily with full confidence in the board exam paper. Solving Class 6 Chemistry objective questions correctly requires a lot of critical & logical thinking. This can occur through our provided CBSE Board Class 6 Chemistry Objective Type Questions with Solutions for all chapters.
Cracking the Objective type questions needs a lot of hard work and practice. Here, we have given the quick links to avail the Chapterwise CBSE Chemistry MCQ Questions for Class 6 with Answers to ace up your preparation.
CBSE Class 6 Chemistry Objective Questions (MCQ) Chapterwise
- Fibre to Fabric
- Changes Around Us
- Sorting Materials into Groups
- Acids, Bases and Salts
- Physical and Chemical Changes
- Weather Climate and Adaptations of Animals to Climate
- Winds, Storms and Cyclones
- Synthetic Fibres and Plastics
- Metals and Non metals
- Matter in our Surroundings
- Is Matter Around us Pure?
- Atoms and Molecules
- Structure of an Atom
- Chemical Equations and Reactions
- Acids, Bases and Salts
- Metals and Non-metals
- Carbon and its Compounds
- Periodic Classification of Elements
We at NCERTBooks.guru guide students to prepare adequately for the tough Class 6 Chemistry MCQ Questions sections & let them help to score great marks in the Exams. Look at the below list of chapter wise CBSE MCQ Questions with Answers for Class 6 Chemistry & practice well.
Fibre to Fabric MCQ Questions
1. Consider the following statements about mixture:
(i) It is made up of pure substances.
(ii) It can be solid, liquid or gas.
(iii) Components do not retain their original properties.
Which of the above statements is/are correct?
(a) (i), (ii), (iii)
(b) (i) and (iii)
(c) (i) and (ii)
(d) (ii) and (iii)
Answer
Answer: (c)
Explanation:
A mixture is made up two or more substances. The pure substances which sire present in a mixture are called components of the mixture (or constituents of the mixture). The components of a mixture retain their original properties. So, a mixture shows the properties of all substances present in it. A mixture can be solid, liquid or gas. For example, soil is a solid mixture, milk is a liquid mixture whereas air is a gaseous mixture. In some cases we can easily see the different substances (or components) present in mixture.
2. Which of the following are correctly matched?
(i) Threshing _______ stalks are beaten to separate grains
(ii) Winnowing _______ method of separating husk from grains
(iii) Hand-picking _______ used to separate undesirable substances
(iv) Sieving _______ used to separate solid mixtures of same size
(a) (i), (ii) and (iv)
(b) (i), (ii) and (iii)
(c) (i), (ii) and (iv)
(d) (i), (ii), (iii) and (iv)
Answer
Answer: (b)
Explanation:
Threshing is the process in which stalks (of wheat, paddy, etc.) are beaten to separate grains from stalks, and the chaff that covers the grains. The method of separation is based on the fact that the ‘stalks’ of the crop plants and the ‘chaff are soft materials whereas the grains themselves are very hard. Winnowing is the method of separating husk from grains with the help of wind. This method is based on the fact that husk is very light whereas wheat grains are comparatively heavy. Hand-picking means to take out by hand. The method of hand-picking is used to separate those substances where one of the components is in small quantify. The method of hand-picking is used to separate undesirable substances such as small pieces of stones from wheat, rice and pulses. Sieving is used to separate those solid mixtures which have components of different sizes.
3. Consider the statements about saturated solutions :
(i) No more substance can be dissolved at given temperature.
(ii) Solubility of a substance refers to saturated solution.
(iii) It contains maximum amount of substance
Which of the above statements is/are correct?
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iii)
(d) (i), (ii) and (iii)
Answer
Answer: (a)
Explanation:
A given quantity of water can dtssolve only a certain maximum amount of a substance (at particular temperature). A solution in which no more substance can be dissolved at that temperature, is called a saturated substance. A saturated solution contains the maximum amount of substance which can be dissolved in it at a given temperature. The solubility of a substance refers to its saturated solution.
4. The process of distillation can be used to separate a certain type of a mixture into their constituents. This process consists of:
(a) Evaporation followed by filtration.
(b) Filtration followed by evaporation.
(c) Evaporation followed by condensation.
(d) Condensation followed by evaporation.
Answer
Answer: (c)
Explanation:
Distillation is the process of heating water to form water vapour, and then cooling water vapour (steam) to get back liquid water. The changing of water into water vapour on heating, is called evaporation (or boiling). And the changing back of hot water vapour (or steam) into liquid water on cooling, is called condensation. Thus distillation involves two processes: evaporation (or boiling) followed by condensation.
Changes Around Us MCQ Questions
1. Consider the following statements about reversible changes:
(i) It is a change which can be reversed to form original substance.
(ii) Change of state is a reversible change.
Which of the above statements is/are correct?
(a) Only (i)
(b) Only (ii)
(c) Both (i) and (ii)
(d) Neither (i) nor (ii)
Answer
Answer: (c)
Explanation:
A change which can be reversed to form the original substance is called a reversible change. When ice changes into water, then there is a change from solid state to liquid state. And when water changes into steam, then there is a change from liquid state to gaseous state. So, in general we can say that: change of state is a reversible change.
2. Which of the following are irreversible changes?
1. Expansion on heating
2. Formation of curd
3. Grinding of wheat to form flour
4. Cooking of food
Which of the above statements is/are correct?
(a) 2, 1 and 4
(b) 2, 3, 4
(c) 1,2 and 3
(d) 1,2, 3 and 4
Answer
Answer: (b)
Explanation:
A change which cannot be reversed to form the original substance (or substances) is called an irreversible change. A very small quantity of curd is added to warm milk. It is kept aside for few hours. During this time, milk changes into curd. This curd cannot be changed back into milk by any means. So, it is a irreversible change. The flour cannot be converted back into wheat grains. So, the grinding of wheat to form flour is an irreversible change. The cooked food cannot be converted back into raw food materials. So, cooking is an irreversible change. The increase of size on heating is called expansion. It is called reversible change because when the hot object is cooled, it decreases in size and comes back to the original size.
Sorting Materials into Groups MCQ Questions
1. The advantage/s of classification is/are:
1. It easier to locate them.
2. It helps in understanding them.
Which of the above statements is/are correct?
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (c)
Explanation:
We classify the objects because it gives us the following advantages:
(i) The classification of objects into groups makes it easier to locate them and work with them.
(ii) The classification of objects into groups helps in understanding them. This is because if we know properties of one member of group, we can get an idea of the properties of the other members of the group.
2. Which of the following properties will be considered for the classification of the materials?
1. Hardness or softness
2. Solubility or insolubility
3. Transparency, translucency or opaqueness
4. Shape
5. Appearance
Which of the above statements is/are correct?
(a) 1,2,3,4,5
(b) 1,3,4,5
(c) 1,2,4,5
(d) 1,2,3,4
Answer
Answer: (b)
Explanation:
The classification of the various objects can be done on the basis of their similarities or dissimilarities. The materials are classified on the basis of property such as appearance, hardness, solubility, heaviness or lightness with respect to water transparency, translucency or opaqueness, use, colour, shape, size, texture, etc.
Acids, Bases and Salts MCQ Questions
1. Which of the following is/are correctly matched?
1. Mineral acid _________ obtained from minerals
2. Natural acid _________ obtained from fruits and vegetables
3. Alkali _________ non-soluble bases,
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (a)
Explanation:
The word ‘acid’ is derived from the Latin word ‘acidus’ which means ‘sour’. The acids which occur in fruits and vegetables, etc. are called naturally-occurring acids. The naturally-occurring acids are weak acids. These acids are also called organic acids. The acids obtained from minerals are called mineral acids. Mineral acids are called inorganic acids. The bases which are soluble in water are called alkalis.
2. Which of the following is/are correctly matched?
1. Sour milk _________ lactic acid
2. Grapes _________ tartaric acid
3. Bee sting _________ maleic acid
4. Vinegar _________ acetic acid
(a) 1,2 and 3
(b) 1, 2 and 4
(c) 2, 3 and 4
(d) 1, 2, 3 and 4
Answer : b
Explanation:
| Name of acid | Found in |
| Acetic acid | Vinegar |
| Formic acid | Ant’s sting |
| Citric acid | Citrus fruits such as oranges, lemons, etc |
| Lactic acid | Curd, sour milk |
| Oxalic acid | Spinach |
| Ascorbic acid | Amla, Citrus fruits (Vitamin C) |
| Tartaric acid | Tamarind, grapes, unripe mangoes, etc. |
| Apples(green) | Maleic acid |
Answer
Answer: (b)
3. Which of the following are correctly matched?
1. Hydrochloric acid _________ extracting glue from bones
2. Nitric acid _________ Refining gold and silver
3. Sulphuric acid _________ textile indus try
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (a)
Explanation:
Some important uses of acid are:
- Hydrochloric acid for cleaning tiles, toilets; for the preparation of chlorides and chlorine gas; for cleaning iron sheets before galvanization: for extracting glue from bones; In textile industry for dyeing.
- Nitric acid for preparing fertilizers, explosives and drugs; in for refining of gold and silver.
- Sulphuric acid for preparing fertilizers, detergents, plastics, synthetic fibres; in petroleum industry for refining; in cleaning metal surfaces before electroplating.
4. Which of the following is/are correct for indicators?
1. Litmus is extracted from lichen.
2. Turmeric is yellow in acidic solution.
3. China rose indicator is magenta in water. Which of the above statements are correct?
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (a)
Explanation:
Special type of substances are used to test whether a substance is acidic or basic. These substances are known as indicators. The indicators change their colour when added to a solution containing an acidic or a basic substance. The most commonly used natural indicator is litmus. It is extracted from lichens. When added to . an acidic solution, it turns red and when added to a basic solution, it turns blue. Turmeric is yellow in neutral solutions, yellow in acidic solutions and red in basic solutions. China rose indicator in water is pink, magenta in acidic solution and green in basic solution.
5. Which of the following is/are correct for neutralisation reaction?
1. It is an endothermic reaction.
2. Its products are salt and water.
Which of the above statements is/are correct?
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (b)
Explanation:
When an acidic solution is mixed with a basic solution, both the solutions neutralise the effect of each other. When an acid solution and a base solution are mixed in suitable amounts, both the acidic nature of the acid and the basic nature of the base are destroyed. The resulting solution is neither acidic nor basic. The reaction between an acid and a base is known as neutralisation. Salt and water are produced in this process with the evolution of heat. Acid+Base-> Salt+Water (Heat is evolved). Heat is evolved; therefore, it is an exothermic reaction.
Physical and Chemical Changes MCQ Questions
1. Which of the following are correctly matched?
1. Dissolution _________ solute gets dissolved in a solvent.
2. Exothermic _________ heat in absorbed.
3. Reversible change _________ reactants can be obtained.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (b)
Explanation:
Dissolution is a process by which any solute gets dissolved in a solvent. Exothermic reactions are those reactions during which heat is evolved. Endothermic reaction is that reaction during which heat is absorbed. The change which can be reversed by reversing the conditions is called reversible change. Example—glowing of a bulb.
2. Which of the following is/are correct for a physical change?
1. Only physical properties change.
2. Large amount of heat is absorbed or evolved.
Which of the above statements is/are correct?
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (a)
Explanation:
Properties such as shape, size, colour and state of a substance are called its physical properties. A change in which a substance undergoes a change in its physical properties is called a physical change. A physical change is generally reversible. In such a change no new substance is formed. Physical changes are temporary and reversible. No or very small amount of energy is either absorbed or evolved during a physical change.
3. Which of the following is not a chemical change?
(a) Burning of a candle.
(b) Cooking a food.
(c) Sublimation.
(d) Germination of seeds.
Answer
Answer: (c)
Explanation:
A change in which one or more new substances are formed is called a chemical change. A chemical change is also called a chemical reaction. Chemical changes are very important in our lives. All new substances are formed as a result of chemical changes. For example, digestion of food in our body, ripening of fruits, fermentation of grapes, etc., happen due to series of chemical changes. A change in which a substance undergoes a change in its physical properties is called a physical change.. During sublimation, a solid substance when heated changes into vapour form, and the vapour on cooling gives back the substance in solid form.
4. Rusting of iron can be prevented by:
1. Painting
2. Galvanisation
3. Electrolytic refining
4. Alloying
Which of the above are correct?
(a) 1,2 and 3
(b) 1,2 and 4
(c) 2, 3 and 4
(d) 1, 2, 3 and 4
Answer
Answer: (b)
Explanation:
The process of rusting can be represented by the following equation: Iron (Fe) + Oxygen (02, from the air) + water (H2O) => rust (iron oxide Fe2O3 ) For rusting, the presence of both oxygen and water (or water vapour) is essential. In fact, if the content of moisture in air is high, which means if it is more humid, rusting becomes faster. This process of depositing a layer of zinc on iron is called galvanisation. The iron pipes we use in our homes to carry water are galvanised to prevent rusting, painting, greasing, oiling, tinning and cathodic protection can be used to prevent rusting of iron.
5. Which of the following is/are correct for crystallisation?
1. It is a physical change.
2. It is used to obtain pure substance.
Which of the above statements is/are correct?
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (c)
Explanation:
The salt obtained in this manner is not pure and the shape of its crystals cannot be seen clearly. However, large crystals of pure substances can be formed from their solutions. Such a process is called crystallisation. It is an example of a physical change.
Weather Climate and Adaptations of Animals to Climate MCQ Questions
1. Which of the following is/are correct for weather?
1. Its changes over a long period.
2. Temperature, humidity, rainfall are elements of weather.
3. Study of weather is called meteorology.
Which of the above statements is/are correct?
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (b)
Explanation:
The short-time atmospheric conditions at a particular place and time with respect to temperature, relative humidity, rainfall, wind speed, etc. is called the weather at that place. The temperature, humidity, rainfall, wind speed, etc. are called elements of weather. The science of the study of weather is called meteorology
2. Which of the following is/are benefits of weather forecasting?
1. It allows people to prepare for bad weather.
2. It helps in the planning of daily activities.
Which of the above statements is/are correct?
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (c)
Explanation:
Weather forecast is based on the data collected by weather balloons,satellite, photographs of the cloud formation etc. Weather forecasting is helpful to people in the following ways:
1. It allows people to prepare for bad weather.
2. It helps in the planning of daily activities.
3. Which of the following is/are correct for climate?
1. Variations that occur on day-to-day basis.
2. It is the average weather condition.
3. Climate chart is annual record of average temperature and rainfall.
Which of the above statements is/are correct?
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (b)
Explanation:
The records of the weather have been preserved for the past several decades. These help us to determine the weather pattern at a place. The average weather pattern taken over a long time, say 25 years, is called the climate of the place. The annual record of long term average temperature and rainfall at a particular place is called climate chart.
4. Which of the geographical factors affect climate?
1. Distance from sea.
2. Direction of prevailing wind
3. Mountains
4. Distance from equator.
Which of the above statements are correct?
(a) 1,2 and 3
(b) 1,2 and 4
(c) 2, 3 and 4
(d) 1, 2, 3 and 4
Answer
Answer: (d)
Explanation:
The sea affects the climate. Coastal areas are cooler and wetter than the inland areas. Warm ocean currents make the climate warm and wet. The direction of the prevailing winds from the sea/ocean side bring rain to coastal areas and dry weather to inland areas. Mountains receive more rainfall than low lying areas because the temperature on top of mountains is lower than sea level. The distance from the equator affects the climate of a place. The equator receives the more sunlight than anywhere else on the earth.
5. Which of the following is/are correct for adaptation?
1. It is a tendency of an organism to develop certain features.
2. Permanent adaptation is inherited.
Which of the above statements is/Eire correct?
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (a)
Explanation:
Animals are adapted to survive m the conditions in which they live. Animals living in very cold and hot climate must possess special features to protect themselves against the extreme cold or heat. Adaption is of two types- permanent- has a genetic basis and it causes permanent changes in the individuals: temporary- is a short term adaptation and is not inherited.
6. Which of the following are correctly matched?
1. Penguin _______ polar region.
2. Arboreal _______ animals live on tress.
3. Monkey _______ tropical forest
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (d)
Explanation:
The lion-tailed macaque (also called Beard ape) lives in the rainforests of Western Ghats. Its most outstanding feature is the silver-white mane, which surrounds the head from the cheeks down to its chin. Many animals are adapted to living on the trees (arboreal). Red-eyed frog has developed sticky pads on its feet to help it climb trees on which it lives. To help them live on the trees, monkeys have long tails for grasping branches.
Winds, Storms and Cyclones MCQ Questions
1. Which of the following are correctly matched?
1. Troposphere _______ clouds, rain
2. Stratosphere _______ ozone layer.
3. Mesosphere _______ temperature increases with altitude.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1,2 and 3
Answer
Answer: (a)
Explanation:
The main layers of the atmosphere are: Troposphere—most of the atmospheric air is present in troposphere. Clouds, rain and snow are seen in troposphere.
Stratosphere- there is very little water vapour. Supersonic aircraft fly in this layer. Ozone layer lies in central zone of stratosphere. Thermosphere-temperature increases with altitude.
2. Which of the following is/are correct about atmospheric pressure?
1. Pressure exerted by air on the earth.
2. It creates winds.
3. It does not influence weather.
Which of the above statements are correct?
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1,2 and 3
Answer
Answer: (a)
Explanation:
The pressure exerted by air at any point on the earth is called atmospheric pressure. Air pressure is important for the following reasons:
- It creates winds: when air moves, it becomes wind. The difference in atmospheric pressure gives rise to the wind system of the earth.
- It influences weather: changes in weather are related to changes in atmospheric pressure.
- Weather forecasting: changes in air pressure give important clues for forecasting weather.
3. Which of the following factors is/are not affected by speed and direction of wind?
(a) Location on the earth
(b) Revolution of earth
(c) Local conditions
(d) Height from ground
Answer
Answer: (b)
Explanation:
Blowing or moving air is called wind. The speed of wind may vary from 5-10 km/h (gentle breeze) to 700-800 km/h (tornado). The speed and direction of wind is affected by the following factors : Location on the earth Rotation of the earth Local conditions Height from the ground
4. What is/are the conditions required for the formation of thunderstorm?
1. Moisture
2. Rapidly rising warm air
3. Sea breeze or mountains.
Which of the above statements are correct?
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (d)
Explanation:
Thunderstorms develop in hot and humid tropical regions. Formation of a thunderstorm requires the following:
- Moisture
- Rapidly rising warm air
- Sea breeze or mountains
The swift movement of the falling water along with rising warm air, producing sound, lightning, heavy rain, and strong wind is called thunderstorm.
5. Which of the following is/are correct for cyclones?
1. They are low-pressure systems with high speed winds.
2. The wind speed is anticlockwise in Southern Hemisphere.
3. Tropical cyclones are more powerful than temperate cyclones.
Which of the above statements are correct?
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (c)
Explanation:
A small low-pressure system with very high speed winds revolving around it is called cyclone. The high speed winds revolve in anti-clock-wise direction in Northern Hemisphere and in clockwise direction in the Southern Hemisphere. Factors like wind speed, wind direction, temperature and humidity contribute to the development of cyclones. The centre of a cyclone is a calm area. It is called the eye of the cyclone. Cyclones that develop in the tropical region are different from those in the temperate latitudes. In general, tropical cyclones are more powerful than temperate cyclones.
Synthetic Fibres and Plastics MCQ Questions
1. Which of the following are correctly matched?
1. Polymer _______ long chain molecule
2. Synthetic fibre _______ man-made fibre
3. Nonbiodegradable _______ decompose to harm less substances.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (a)
Explanation:
Polymer-long chain-like molecule consisting of a large number of molecules joined to each other by chemical bonds.
Non biodegradable- substances which do not decompose to harmless substances by the action of air, water and bacteria.
Synthetic fibre- man-made fibres from simple, small molecules.
2. Which of the following is/are correct about polymers?
1. It is a long chain like unit consisting of a large number of smaller molecules.
2. Smaller molecules are monomers.
3. Polymers are natural.
Which of the above statements are correct?
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (a)
Explanation:
A long chain-like unit consisting of a large number of smaller molecules joined to each other by chemical bonds is called a polymer. The smaller molecules which combine to form the chain of a polymer are called monomers. The polymers which occur in nature are called natural polymers. For example, cellulose, silk, wool, proteins, etc. are natural polymers. The polymers which are made in laboratories factories from small molecules are called man-made (or synthetic) polymers. For example, nylon, polythene, polyvinyl chloride, rayon and teflon are some synthetic polymers.
3. Which of the following are correctly matched?
1. Rayon used in textile industry.
2. Nylon used for making fishing nets.
3. Polyester used for manufacture of tyre cords.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (a)
Explanation:
Rayon is used—
- in the textile industry for making fabrics;
- in the manufacture of carpets;
- for the manufacture of tyre cord;
- for making bandages and surgical dressings. Nylon is used—
- for the manufacture of iyre cords, fabrics and ropes;
- for making fishing nets and parachute ropes;
- for fabricating sheets, bristles for brushes, for making sarees, socks, neckties;
- for making elastic hosiery;
- for making machine parts.
Polyester is used-
- for manufacturing sarees, dress materials, curtain cloth, etc.
- for making blends with other fibres; eg. grylene with cotton gives terycot, with wool it gives teiywool. Clothes made from blends are more comfortable to wear.
- for making sails for sail boats;
- for making water hoses for fire fighting;
- for making conveyor belts.
4. Which of the following is/are correct of synthetic fibres?
1. They do not get wet.
2. They are not durable.
3. They are cheaper.
Which of the above statements are correct?
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (c)
Explanation:
Some of the characteristic properties of synthetic fibres are :
- Synthetic fibre does not get wet. So, the fabrics made from synthetic fibres dry up quickly.
- Synthetic fibres cure durable i.e, fabrics made from synthetic fibres last long.
- Synthetic fibres can be drawn to very fine thickness. So these are light-weight. As a result, the fabrics made from synthetic fibres are thin and hence light-weight.
- Synthetic fibres are strong, have high tensile strength and are abrasion-resistant. As a result, the fabrics made from synthetic fibres are long lasting and easy to maintain.
- Synthetic fibres are cheaper.
5. Which of the following statement is/are correct for thermoplastics?
1. They are long-chain polymer with cross-linking.
2. They can be processed repeatedly.
3. Polyethene, PVC are examples of thermoplastics.
Which of the above statements are correct?
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (b)
Explanation;
1. Thermoplastics are long chain polymers with
no cross-linking. Heating also does not produce any cross-linking between the chains
2. Thermoplastics can be processed repeatedly Example : Polythene, PVC, Polystyrene, Nylon, Polyesters, etc.
6. Which of the following are correctly matched?
1. Polyethene _______ thermoplastic polymer.
2. Bakelite _______ thermosetting plastic
3. Melamine _______ thermosetting polymer.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1,2 and 3
Answer
Answer: (d)
Explanation:
Polythene (or polyethylene) is obtained from ethylene under high temperature, high pressure and in the presence of a catalyst. Polythene is a thermoplastic polymer. So, it can be moulded into any shape and any number of times. Bakelite is a thermosetting plastic. Once set into a shape, bakelite does not melt/soften and retains its shape. Bakelite is obtained by reacting phenol with formaldehyde in the presence of a catalyst Melamine is also a thermosetting polymer. It is hard and a high polish polymer. Melamine is used for making unbreakable dinner-ware, and decorative objects rative objects.
Metals and Non-metals MCQ Questions
1. Which of the following are correctly matched?
1. Metals _______ lead, arsenic
2. Non-metals _______ carbon, sulphur
3. Metalloids _______ boron, silicon
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (b)
Explanation:
Metals: Iron, Copper, Gold, Silver, Aluminium, Zinc, Lead are some commonly used metals.
Non-metals: Hydrogen, Oxygen, Nitrogen, Carbon, Sulphur, Phosphorus, Chlorine, Bromine, Iodine are commonly used non-metals
Metalloids: Boron, Silicon, Arsenic and Germanium are some metalloids.
2. Which of the following is/are correct for metals?
1. They are solids.
2. They have metallic lustre.
3. They are hard except sodium and gallium.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1,2 and 3
Answer
Answer: (b)
Explanation:
1. Physical state: Under normal pressure, all metals except mercury are solid at room temperature. Mercury is liquid at room temperature.
2. Colour : Most metals except gold and copper are silvery grey in colour. Copper is reddish-brown and gold is golden yellow.
3. Appearance : All metals are shiny. The characteristic shine of metals is called metallic lustre. Thus, all metals have metallic lustre. Metals can be easily polished.
4. Hardness : Most metals are hard except sodium and potassium. Sodium and potassium metals can be easily cut with a knife. Osmium is hard enough to scratch glass.
3. Which of the following are correctly matched?
1. Graphite _______ good conductor
2. Caesium _______ high melting point
3. Diamond _______ hardest substance
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1,2 and 3
Answer
Answer: (c)
Explanation:
Graphite is the only non-metal which conducts heat and electricity. Caesium metal have low melting point that it melts even when kept on palm. Diamond is the hardest substance known on earth.
4. The reaction Zinc + copper sulphate solution’ zinc
sulphate solution + copper indicates that the metal which is placed lower in the reactivity series is :
(a) Zinc
(b) Copper
(c) Zinc (II) ion
(d) Copper (II) ion
Answer
Answer: (b)
Explanation:
The reactivity series of metals is an arrangement of metals in vertical column in order of decreasing reactivity The reactivity series is also called activity series of metals. In the activity series, the most reactive metal is placed at the top whereas the least reactive metal is placed at the bottom. The more reactive metal has greater tendency to form compounds. So, such metals are found only in the form of their compounds. Less reactive metals such as silver, gold and platinum are found in the free state in the earth’s crust.
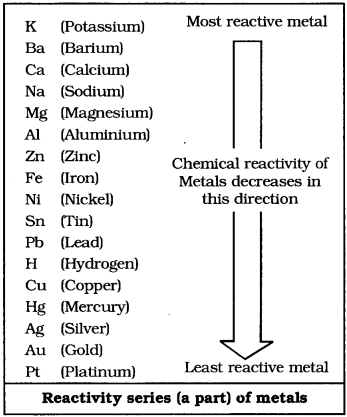
5. Which of the following are correctly matched?
1. Sulphur _______ manufacture of gun powder
2. Phosphorus _______ manufacture of match sticks
3. Oxygen _______ supporter of combustion
4. Hydrogen _______ for sterilizing water
(a) 1, 2 and 3
(b) 2, 3 and 4
(c) 1,2 and 4
(d) 1,2, 3 and 4
Answer
Answer: (a)
Explanation:
Sulphur is used in manufacture of gun powder, sulphuric acid, in vulcanization of rubber. Phosphorus is used for the manufacture of match sticks, rat poison, phosphoric acid and fertilizers. Oxygen is supporter of combustion and used for respiration by living organisms. Hydrogen is used as a fuel, for manufacturing ammonia, hydrogen chloride, vegetable ghee by hydrogenation of oils. Chlorine is used for bleaching and sterilizing water.
Matter in our Surroundings MCQ Questions
1. Which of the following is/are the characteristics of matter?
1. Particles of matter have space between them.
2. Particles of matter are stationaiy.
3. Particles of matter attract each other.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (c)
Explanation:
Characteristics of particles of matter:
- Particles of matter have space between them.
- Matter is made up of particles.
- Particles of matter are continuously moving.
- Particles of matter attract each other.
2. Which of the following is/are correct for effect of temperature?
1. The kinetic energy of the particles increases.
2. The energy supplied by heat overcomes the forces of attraction between the particles.
3. The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its boiling point.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (a)
Explanation:
On increasing the temperature of solids, the kinetic energy of the particles increases. Due to the increase in kinetic energy, the particles start vibrating with greater speed. The energy supplied by heat overcomes the forces of attraction between the particles. The particles leave their fixed positions and start moving more freely. A stage is reached when the solid melts and is converted to a liquid. The minimum temperature at which a solid melts to become a liquid at the atmospheric pressure is called its melting point. The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point.
3. Which of the following is/are correct about latent heat?
1. The word latent means hidden.
2. The amount of heat energy that is required to change 1 kg. of a liquid into gas.
(a) Only 1
(b) only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (a)
Explanation:
The word latent means hidden. The amount of heat energy that is required to change 1 kg. of a solid into liquid at atmospheric pressure at its melting point is known as the latent heat of fusion. So, particles in water at 0°C (273 K) have more energy as compared to particles in ice at the same temperature.
4. Which of the following are correctly matched?
1. Sublimation _______ change from solid to gas.
2. Deposition solid _______ direct change of gas to
3. Pressure and reducing temperature _______ solidify gases.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1,2 and 3
Answer
Answer: (a)
Explanation:
A change of state directly from solid to gas without changing into liquid state is called sublimation and the direct change of gas to solid without changing into liquid is called deposition. Applying pressure and reducing temperature can liquefy gases, dry ice, etc. Thus, we can say that pressure and temperature determine the state of a substance, whether it will be solid, liquid or gas.
5. Which of the following is/are correct for evaporation?
1. Change of a liquid into vapours at any temperature below its boiling point.
2. Increase in temperature decreases evaporation.
3. Decrease in humidity increases evaporation,
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (c)
Explanation:
The phenomenon of change of a liquid into vapours at any temperature below its boiling point is called evaporation. The rate of evaporation increases with-
- an increase of surface area: If the surface area is increased, the rate of evaporation increases.
- an increase of temperature: With the increase of temperature, more number of particles get enough kinetic energy to go into the vapour state.
- a decrease in humidity: Humidity is the amount of water vapour present in air. The air around us cannot hold more than a definite amount of water vapour at a given temperature. If the amount of water in air is al-ready high, the rate of evaporation decreases.
- an increase in wind speed: It is a common observation that clothes dry faster on a windy day. With the increase in wind speed, the particles of water vapour move away with the wind, decreasing the amount of water vapour in the surrounding.
6. What is the correct order of force of attraction?
(a) Solids>gases > liquids
(b) Solids> liquids > gases
(c) Liquid> gases > solids
(d) Gases>liquids > solid
Answer
Answer: (b)
Explanation:
Matter is made up of small particles. The matter around us exists in three states- solid, liquid and gas. The forces of attraction between the particles are maximum in solids, intermediate in liquids and minimum in gases. The space in between the constituent particles and kinetic energy of the particles are minimum in the case of solids, intermediate in liquids and maximum in gases.
Is Matter Around us Pure? MCQ Questions
1. Which of the following is/are correct for mixtures?
1. Mixtures are constituted by more than one kind of pure form of matter.
2. Alloys are mixtures of two or more metals or a metal and a non-metal and cannot be separated into their components by physical methods.
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (c)
Explanation:
Mixtures are constituted by more than one kind of pure form of matter. Alloys are mixtures of two or more metals or a metal and a non-metal and cannot be separated into their components by physical methods. But still, an alloy is considered as a mixture because it shows the properties of its constituents and can have variable composition. For example, brass is a mixture of approximately 30% zinc and 70% copper.
2. Which of the following is/are correct for solutions?
1. A solution is a heterogeneous mixture of two or more substances.
2. It can be solid solutions (alloys) and gaseous solutions (air).
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (b)
Explanation:
A solution is a homogeneous mixture of two or more substances. You come across various types of solutions in your daily life. Lemonade, soda water, etc. are all examples of solutions. Usually we think of a solution as a liquid that contains either a solid, liquid or a gas dissolved in it. But, we can also have solid solutions (alloys) and gaseous solutions (air). In a solution there is homogeneity at the particle level.
3. Which of the following are correctly matched?
1. Solvent _______ dissolves the substance.
2. Solute _______ component that is dissolved
3. Tincture of iodine _______ solution of iodine
in water.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (a)
Explanation:
A solution has a solvent and a solute as its components. The component of the solution that dissolves the other component in it (usually the component present in larger amount) is called the solvent. The component of the solution that is dissolved in the solvent (usually present in lesser quantity) is called the solute. A solution of iodine in alcohol known as ‘tincture of iodine’, has iodine (solid) as the solute and alcohol (liquid) as the solvent.
4. Which of the following is/are the characteristics of colloid?
1. A colloid is a heterogeneous mixture.
2. Colloids are big enough to scatter a beam of light.
3. The particles of a colloid can be seen by the naked eye.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (a)
Explanation:
A colloid is a heterogeneous mixture.
- The size of particles of a colloid is too small to be individually seen by naked eyes.
- Colloids are big enough to scatter a beam of light passing through it and make its path visible.
- They do not settle down when left undisturbed, that is, a colloid is quite stable.
5. Which of the following are correctly matched?
1. Dispersed phase _______ dispersed particles.
2. Dispersion medium _______ dispersed phase is suspended.
3. Aerosol _______ fog.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (d)
Explanation:
The components of a colloidal solution are the dispersed phase and the dispersion medium. The solute-like component or the dispersed particles in a colloid form the dispersed phase, and the component in which the dispersed phase is suspended is known as the dispersing medium. Examples of Aerosol : fog, clouds, mist.
6. Which of the following are correctly matched?
1. Coloured dye from black ink _______ evaporation.
2. Cream from milk _______ filtration.
3. Oil and water _______ separating funnel.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (c)
Explanation:
Ink is a mixture of a dye in water. Thus, we can separate the volatile component (solvent) from its non-volatile solute by the method of evaporation. Cream from milk is separated by centrifugation. The principle is that the denser particles are forced to the bottom and the lighter particles stay at the top when spun rapidly. The principle is that immiscible liquids separate out in layers depending on their densities using separating funnel.
7. Which of the following is/are correct about elements?
1. Elements can be normally divided into metals, non-metals and metalloids.
2. Antoine Laurent Lavoisie was the first scientist to use the term ‘element’ in 1661.
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (a)
Explanation:
Robert Boyle was the first scientist to use the term element in 1661. Antoine Laurent Lavoisier (1743-94), a French chemist, was the first to establish an experimentally useful definition of an ‘element’. He defined an ‘element’ as a basic form of matter that cannot be broken down into simpler substances by chemical reactions. Elements can be normally divided into metals, non-metals and metalloids.
8. Which of the following is/are correct for com’ pounds?
1. It is a substance composed of two or more elements.
2. The composition of each new substance is always fixed.
3. It shows the properties of the constituent substances.
Which of the above statements are correct?
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (a)
Explanation:
1. Elements react to form new compounds,
2. The composition of each new substance is al’ ways fixed.
3. The new substance has totally different con; stituent substances and properties.
4. The constituents can be separated only by chemical or electrochemical reactions.
Atoms and Molecules MCQ Questions
1. Which of the following is/are true for Dalton atomic theory?
1. All matter is made up of very tiny particles called atoms.
2. Atoms do not participate in chemical reactions.
3. Atoms are indivisible particles.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (c)
Explanation:
According to Dalton’s atomic theory, all matter, whether an element, a compound or a mixture is | composed of small particles called atoms. The postulates of this theory may be stated as follows:
(i) All matter is made up of very tiny particles , called atoms, which participate in chemical reactions.
(ii) Atoms are indivisible particles, which can not be created or destroyed in a chemical reaction.
(iii) Atoms of a given element are identical in mass and chemical properties.
(iv) Atoms of different elements have different masses and chemical properties.
(v) Atoms combine in the ratio of small whole numbers to form compounds.
(vi) The relative number and kinds of atoms are constant in a given compound.
2. Which of the following is/are correct for law of constant proportion?
1. It was given and stated by Lavoisier.
2. In a chemical substance the elements are always present in definite proportions by mass.
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (b)
Explanation:
Lavoisier, along with other scientists, noted that many compounds were composed of two or more elements and each such compound had the same elements in the same proportions, irrespective of where the compound came from or who prepared it. This law was stated by Proust as “In a chemical substance the elements are always present in definite proportions by mass”.
3. Which of the following is/are correct for atomic mass?
1. It was proposed by Bohr.
2. It was explained by law of constant proportions.
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (b)
Explanation:
The most remarkable concept that Dalton’s atomic theoiy proposed was that of the atomic mass. According to him, each element had a characteristic atomic mass. The theory could explain the law of constant proportions so well that scientists were prompted to measure the atomic mass of an atom.
4. Which of the following is/are correct for molecules?
1. It is a group of two or more atoms that are chemically bonded together.
2. Atoms of only the same element join to form molecules.
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (a)
Explanation:
A molecule is, in general, a group of two or more atoms that are chemically bonded together, that is, tightly held together by attractive forces. A molecule can be defined as the smallest particle of an element or a compound that is capable of an independent existence and shows all the properties of that substance. Atoms of the same element or of different elements can join together to form molecules.
5. Which of the following are correctly matched?
1. Argon- monoatomic
2. Phosphorus polyatomic
3. Chlorine-diatomic
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (c)
Explanation:
| Type of Element Non-Metal | Name | Atomicity |
| Non-Metal | Argon | Monoatomic |
| Helium | Monoatomic | |
| Oxygen | Diatomic | |
| Hydrogen | Diatomic | |
| Nitrogen | Diatomic | |
| Chlorine | Diatomic | |
| Phosphorus | Tetra-atomic | |
| Sulphur | Poly-atomic |
6. Which of the following is/are correct for ions?
1. The charged species are known as ions.
2. A negatively charged ion is called an cation
3. A group of atoms carrying a charge is known as a polyatomic ion.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (c)
Explanation:
Compounds composed of metals and non-metals contain charged species. The charged species are known as ions. Ions may consist of a single charged atom or a group of atoms that have a net charge on them. An ion can be negatively or positively charged. A negatively charged ion is called an ‘anion’ and the positively charged ion, a ‘cation’. A group of atoms carrying a charge is known as a polyatomic ion.
Structure of an Atom MCQ Questions
1. Which of the following are correctly matched?
1. Electron _______ J.J Thomson
2. Proton _______ Dalton.
3. Neutron _______James Chadwick
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (c)
Explanation:
It was known by 1900 that the atom was indivisible particle but contained at least one subatomic particle – the electron identified by J.J. Thomson. Even before the electron was identified, E. Goldstein in 1886 discovered the presence of new radiations in a gas discharge and called them canal rays. James Chadwick discovered neutron.
2. Which of the following is/are correct for Thomson model of an atom?
1. An atom consists of a positively charged sphere and the electrons are embedded in it.
2. The negative and positive charges are unequal in magnitude. So, the atom as a whole is electrically neutral.
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (a)
Explanation:
Thomson proposed that:
(i) An atom consists of a positively charged sphere and the electrons are embedded in it.
(ii) The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral. Although Thomson’s model explained that atoms are electrically neutral.
3. Which of the following is/are correct for observation of Rutherford experiment?
1. Most of the fast moving a-particles passed straight through the gold foil.
2. Some of the a-particles are deflected by the foil by small angles.
3. Surprisingly one out of every 1200 particles appear to rebound.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (a)
Explanation:
In the experiment, fast moving alpha (α)-particles were made to fall on a thin gold foil.
- He selected a gold foil because he wanted as thin a layer as possible. This gold foil was about 1000 atoms thick.
- α-particles are doubly-charged helium ions. Since they have a mass of 4 u, the fast-moving α-particles have a considerable amount of energy.
- It was expected that α-particles would be deflected by the sub-atomic particles in the gold atoms. Since the α-particles were much heavier than the protons, he did not expect to see large deflections.
The following observations were made:
(i) Most of the fast moving a-particles passed straight through the gold foil.
(ii) Some of the a-particles were deflected by the foil by small angles.
(iii) Surprisingly one out of every 12000 particles appeared to rebound.
4. Which of the following is/are true for electron distribution in shells?
1. It was suggested by Bohr.
2. The maximum number of electrons that can be accommodated in the outermost orbit is 8.
3. The maximum number of electrons present in a shell is given by the formula 2n(square).
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (b)
Explanation:
The distribution of electrons into different orbits of an atom was suggested by Bohr and Bury. The following rules are followed for writing the number of electrons in different energy levels or shells:
(i) The maximum number of electrons present in a shell is given by the formula (2n)2 , where ‘n’ is the orbit number or energy level index.
(ii) The maximum number of electrons that can be accommodated in the outermost orbit is 8.
(iii) Electrons are not accommodated in a given shell, unless the inner shells are filled. That is, the shells are filled in a step wise manner.
5. Which of the following are correctly matched?
| Element | Valency |
| 1. Neon | zero |
| 2. Nitrogen | five |
| 3. Phosphorus | three decimal five |
| 4. Potassium | one |
(a) 1,2 and 3
(b) 1, 2 and 4
(c) 1,3 and 4
(d) 1, 2, 3 and 4
Answer
Answer: (c)
Explanation:
| Element | Atomic number | Valency |
| Hydrogen | 1 | 1 |
| Helium | 2 | 0 |
| Lithium | 3 | 1 |
| Beryllium | 4 | 2 |
| Boron | 5 | 3 |
| Carbon | 6 | 4 |
| Nitrogen | 7 | 3 |
| Oxygen | 8 | 2 |
| Fluorine | 9 | 1 |
| Neon | 10 | 0 |
| Sodium | 11 | 2 |
| Magnesium | 12 | 3 |
| Aluminium | 13 | 3 |
| Silicon | 14 | 4 |
| Phosphorus | 15 | 3.5 |
| Sulphur | 16 | 2 |
| Chlorine | 17 | 1 |
| Argon | 18 | 0 |
| Potassium | 19 | 1 |
| Calcium | 20 | 2 |
6. Which of the following are correctly matched?
1. Mass number _______ denoted by M
2. Atomic number _______ denoted by z
3. Isotopes _______ same atomic number
4. Isobar _______ same atomic mass
(a) 1 and 4
(b) 1, 2 and 3
(c) 1, 3 and 4
(d) 1,2 and 4
Answer
Answer: (c)
Explanation:
Atomic number is the number of protons of an atom, which determines its atomic number. It is denoted by ‘Z’. the atomic number is defined as the total number of protons present in the nucleus of an atom, the mass of an atom resides in its nucleus. The mass number is defined as the sum of the total number of protons and neutrons present in the nucleus of an atom. It is denoted by A’. Isotopes are defined as the atoms of the same element, having the same atomic number but different mass numbers. Atoms of different elements with different atomic numbers, which have the same mass number, are known as isobars.
Chemical Equations and Reactions MCQ Questions
1. Which of the following are the observations of the chemical reaction?
1. Change in state
2. Evolution of a gas
3. Change in colour
4. Change in temperature
(a) 1, 2 and 3
(b) 1,2 and 4
(c) 1,3 and 4
(d) 1,2, 3 and 4
Answer
Answer: (d)
Explanation :
We can say that any of the following observations helps us to determine whether a chemical reaction has taken place or not :
- Change in state
- Change in colour
- Evolution of a gas
- Change in temperature.
As we observe the changes around us, we can see that there are many chemical reactions taking
2. Which of the following are correctly matched?
1. Reactant _______ substance which undergoes change
2. Product _______ new substance
3. Chemical reaction _______ simplest form
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (a)
Explanation:
The description of a chemical reaction in a sentence form is quite long. It can be written in a shorter form. The simplest way to do this is to write it in the form of a word equation. The substances that undergo chemical change in the reaction are the reactants. The new substance is formed during the reaction, is a product.
3. Which of the following is/are correct for a balanced chemical equation?
1. It is based on law of conservation of mass.
2. The physical state makes the chemical reaction less informative.
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (a)
Explanation:
The law of conservation of mass states that mass can neither be created nor destroyed in a chemical reaction. That is, the total mass of the elements present in the products of a chemical reaction has to be equal to the total mass of the elements present in the reactants. In other words, the number of atoms of each element remains the same, before and after a chemical reaction. To make a chemical equation more informative, the physical states of the reactants and products are mentioned along with their chemical formulae. The gaseous, liquid, aqueous and solid states of reactants and products are represented by the notations.
4. Which of the following are correctly matched?
| Symbol | state | |
| 1. | aq | soluble in alcohol |
| 2. | 1 | liquid |
| 3. | S | solid |
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (b)
Explanation:
To make a chemical equation more informative, the physical state of the reactants and products are mentioned along with their chemical formulae. The gaseous, liquid, aqueous and solid states of reactants and products are represented by the notations (g), (1), (aq) and (s), respectively. The word aqueous (aq) is written if the reactant or product is present as a solution in water.
5. Which of the following are correctly matched?
1. Combination reaction _______ formation of single product
2. Decomposition reaction _______ break down of single entity
3. Thermal decomposition heat is used
4. Displacement reaction _______ based on reactivity series
(a) 1, 2 and 3
(b) 1,2 and 4
(c) 1,3 and 4
(d) 1,2, 3 and 4
Answer
Answer: (d)
Explanation:
A reaction in which a single product is formed from two or more reactants is known as a combination reaction. A large amount of heat is evolved. This makes the reaction mixture warm. Reactions in which heat is released along with the formation of products are called exothermic chemical reactions. When a single reactant breaks down to give simpler products, it is a decomposition reaction. When a decomposition reaction is carried out by heating, it is called thermal decomposition. In displacement reaction, the higher reactive metal displaces the less reactive metal.
6. Which of the statements about the reaction below are incorrect?
2PbO(s) + C(s) → 2Pb(s) + CO2(g)
1. Lead is getting reduced.
2. Carbon dioxide is getting oxidised.
3. Carbon is getting oxidised.
4. Lead oxide is getting reduced.
(a) 1 and 2
(b) 1 and 3
(c) 1,2 and 3
(d) All the above
Answer
Answer: (b)
Explanation:
Reactions also involve the gain or loss of oxygen or hydrogen by substances. Oxidation is the gain of oxygen or loss of hydrogen. Reduction is the loss of oxygen or gain of hydrogen. Lead is losing oxygen, it is getting reduced. Carbon is gaining oxygen: it is getting oxidised.
Acids, Bases and Salts MCQ Questions
1. Which of the following statements is/are correct for litmus?
1. Litmus solution is a purple dye.
2. It is extracted from lichen.
3. In neutral solution, it remains colourless.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer: a
Answer
Answer: (a)
Explanation:
Litmus solution is a purple dye, which is extracted from lichen, a plant belonging to the division Thallophyta, and is commonly used as an indicator. When the litmus solution is neither acidic nor basic, its colour is purple.
2. Which of the following is/are correct for olfactory indicators?
1. Their colour changes with acid or base.
2. Onion, vanilla or clove are examples.
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (b)
Explanation:
There are some substances whose odour changes in acidic or basic media. These are called olfactory indicators. Olfactory indicators can be used in the laboratory to test whether a solution is abase or an acid, a process called olfactory titration. Onion, clove oil and vanilla extract are examples of olfactory indicators.
3. Which of the following are correctly matched?
1. Acid + salt _______ metal+ hydrogen
2. Acid+ metal carbonate _______ salt+carbon dioxlde+ water
3. Metal oxide+acid _______ salt + water
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (b)
Explanation:
The metal displaces hydrogen atoms from the adds as hydrogen gas and forms a compound called a salt. Thus, the reaction of a metal with an acid can be summarised as – Acid + Metal → Salt + Hydrogen gas. All metal carbonates and hydrogen carbonates react with acids to give a corre-sponding salt, carbon dioxide and water. Thus, the reaction can be summarised as-
Metal carbonate/Metal hydrogen carbonate + Acid → Salt + Carbon dioxide + Water
The general reaction between a metal oxide and an acid can be written as – Metal oxide + Acid → Salt + Water.
4. Which of the following is/are correct for diluting acid?
1. Adding acid to water by stirring.
2. Adding water to add by stirring
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (a)
Explanation:
The process of dissolving an acid or a base in water is a highly exothermic one. The acid must always be added slowly to water with constant, stirring. If water is added to a concentrated acid, the heat generated may cause the mixture to splash out and cause bums.
5. Which of the following is/are correct for pH?
1. A scale for measuring hydronium ion concentration.
2. Values less than 7 on the pH scale represent an acidic solution.
3. As the pH value increases from 7 to 14, it represents an increase in hydrogen ion concentration in the solution.
(a) Only 1
(b) Only 2
(c) Only 3
(d) 1,2 and 3
Answer
Answer: (b)
Explanation:
A scale for measuring hydrogen ion concentration in a solution, called pH scale has been developed. The p in pH stands for ‘potenz’ in German, meaning power. On the pH scale we can measure pH generally from 0 (very acidic) to 14 (very alkaline). pH should be thought of simply as a number which indicates the acidic or basic nature of a solution. Higher the hydronium ion concentration, lower is the pH value. The pH of a neutral solution is 7. Values less than 7 on the pH scale represent an acidic solution. As the pH value increases from 7 to 14, it represents an increase in OH- ion concentration in the solution, that is, increase in the strength of alkali.
6. Which of the following are correctly matched?
1. Plants and animals _______ pH range is 7.0 to 7.8
2. Rain water _______ pH is 7.6
3. Tooth decay _______ pH less than 5.5
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (c)
Explanation:
Our body works within the pH range of 7.0 to 7.8. Living organisms can survive only in a narrow range of pH change. When pH of rain water is less than 5.6, it is called acid rain. When acid rain flows into the rivers, it lowers the pH of the river water. The survival of aquatic life in such rivers becomes difficult. Tooth decay starts when the pH of the mouth is lower than 5.5. Tooth enamel, made up of calcium hydroxyapatite (a crystalline form of calcium phosphate) is the hardest substance in the body. It does not dissolve in water, but is corroded when the pH in the mouth is below 5.5. Bacteria present in the mouth produce acids by degradation of sugar and food particles remaining in the mouth after eating.
7. Which of the following are correctly matched?
1. Common salt _______ formed by sodium hydroxide and hydrochloric acid.
2. Brine _______ aqueous solution of sodium chloride
3. Chlor-alkali process _______ formation of sodium chloride
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1,2 and 3
Answer
Answer: (a)
Explanation:
The salt formed by the combination of hydrochloric acid and sodium hydroxide solution is called sodium chloride. When electricity is passed through an aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide. The process is called the chlor-alkali process because of the products formed- chlor for chlorine and alkali for sodium hydroxide.
8. Which of the following are correctly matched?
1. Bleaching powder _______ oxidising agent in chemical industries.
2. Baking powder _______ a mixture of sodium hydrogen carbonate and a mild edible acid.
3. Washing soda remove permanent hardness of water.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (d)
Explanation:
Uses of washing soda (i) Sodium carbonate (washing soda) is used in glass, soap and paper industries, (ii) It is used in the manufacture of sodium compounds such as borax, (iii) Sodium carbonate can be used as a cleaning agent for domestic purposes, (iv) It is used for removing permanent hardness of water. For making baking powder, which is a mixture of baking soda (sodium hydrogen carbonate) and a mild edible acid such as tartaric acid. Bleaching powder is used – (i) for bleaching cotton and linen in the textile industry, for bleaching wood pulp in paper factories and for bleaching washed clothes in laundry; (ii) as an oxidising agent in many chemical industries; and (iii) to make drinking water free from germs.
Metals and Non-metals MCQ Questions
1. Which of the following are correctly matched?
1. Ductility _______ drawn into wire
2. Malleability _______ drawn into sheets
3. Good conductors _______ copper and mercury
4. Non-metals solids or gases
(a) 1, 2 and 3
(b) 1,2 and 4
(c) 1, 3 and 4
(d) 2, 3 and 4
Answer
Answer: (b)
Explanation:
The ability of metals to be drawn into thin wires is called ductility. Gold is the most ductile metal. Metals are good conductors of heat and have high melting points. The best conductors of heat are silver and copper. Lead and mercury are comparatively poor conductors of heat. The non-metals are either solids or gases except bromine which is a liquid. The ability of metals to be drawn into . sheets is called malleability.
2. Which of the following are correctly matched?
1. Mercury _______ liquid at room temperature
2. Iodine _______ non-lustrous
3. Lithium _______ low melting point
4. Graphite _______ good conductor
(a) 1, 2 and 3
(b) 1, 2 and 4
(c) 1, 3 and 4
(d) 2, 3 and 4
Answer
Answer: (c)
Explanation:
All metals except mercury exist as solids at room temperature. Iodine is a non-metal but it is lustrous. Carbon is a non-metal that can exist in different forms. Each form is called an allotrope. Diamond, an allotrope of carbon, is the hardest atural substance known and has a very high melting and boiling point. Graphite, another al- lotrope of carbon, is a conductor of electricity. Alkali metals (lithium, sodium, potassium) are so soft that they can be cut with a knife. They have low densities and low melting points.
3. Which of the following statements is/are correct for metals?
1. They react with oxygen to form metal oxides.
2. All metallic oxides are basic in nature.
3. Metals are’ reducing agents.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (c)
Explanation:
Metal react with oxygen to form metal oxide. Metallic oxides are mostly basic. Metals lose electrons to form positive ions. Metals are good reducing agents. More reactive metals can displace less reactive metals from its salt solution.
4. Which of the following statements is/are correct for anodising?
1. It is a process of forming a thick oxide layer of aluminium.
2. The aluminium oxide coat makes it resistant to further corrosion.
3. It is electrolysed with dilute hydrochloric acid,
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (a)
Explanation:
Anodising is a process of forming a thick oxide layer of aluminium. Aluminium develops a thin oxide layer when exposed to air. This aluminium oxide coat makes it resistant to further corrosion. The resistance can be improved further by making the oxide layer thicker. During anodising, a clean aluminium article is made the anode and is electrolysed with dilute sulphuric acid. The oxygen gas evolved at the anode reacts with aluminium to make a thicker protective oxide layer. This oxide layer can be dyed easily to give aluminium articles an attractive finish.
5. Which of the following metals do/does not react with cold water?
1. Potassium
2. Sodium
3. Magnesium
4. Aluminium
(a) 1 and 2
(b) 2 and 3
(c) 2 and 4
(d) 3 and 4
Answer
Answer: (d)
Explanation:
Metals react with water and produce a metal oxide and hydrogen gas. Metal oxides that are soluble in water dissolve in it to further form metal hydroxide. Metals like potassium and sodium react violently with cold water. In case of sodium and potassium, the reaction is so violent and exothermic that the evolved hydrogen immediately catches fire. Magnesium does not react with cold water. It reacts with hot water to form magnesium hydroxide and hydrogen. Metals like aluminium, iron and zinc do not react either with cold or hot water. But, they react with steam to form the metal oxide and hydrogen.
6. Which of the following statements is/are correct for aqua regia?
1. It is a freshly prepared mixture of concentrated hydrochloric acid and concentrated nitric acid.
2. Hydrochloric acid and nitric acid are in ratio 2:1
3. It can dissolve gold.
(a) 1 and 2
(b) 2 and 3
(c) land 3
(d) 1, 2 and 3
Answer
Answer: (c)
Explanation:
Aqua regia, (Latin for ‘royal water’) is a freshly prepared mixture of concentrated hydrochloric acid and concentrated nitric acid in the ratio of 3:1. It can dissolve gold, even though neither of these acids can do so alone. Aqua regia is a highly corrosive, fuming liquid. It is one of the few re-agents that is able to dissolve gold and platinum.
7. What is/are true for ionic compounds?
1. They are solids.
2. They have low melting and boiling points.
3. They are soluble in water.
4. They are good conductors of electricity.
(a) 1,2 and 3
(b) 1, 2 and 4
(c) 1,3 and 4
(d) 2, 3 and 4
Answer
Answer: (c)
Explanation:
The following general properties for ionic compounds-
(i) Physical nature: Ionic compounds are solids and are somewhat hard because of the strong force of attraction between the positive and negative ions. These compounds are generally brittle and break into pieces when pressure is applied.
(ii) Melting and Boiling points: Ionic compounds have high melting and boiling points (see Table 3.4). This is because a considerable amount of energy is required to break the strong inter-ionic attraction.
(iii) Solubility: Electrovalent compounds are generally soluble in water and insoluble in solvents such as kerosene, petrol, etc.
(iv) Conduction of Electricity: The conduction of electricity through a solution involves the movement of charged particles.
8. Which of the following Eire correctly matched for metals?
1. High reactivity _______ electrolysis of molten ore
2. Medium reactivity _______ roasting
3. High reactivity _______ calcination
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1,2 and 3
Answer : c
Explanation:
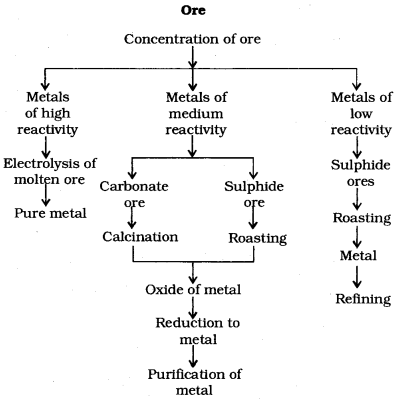
Answer
Answer: (b)
Carbon and its Compounds MCQ Questions
1. Which of the following is correct order of melting points?
(a) Methane > choloroform > ethanol > acetic acid
(b) Methane > acetic acid > ethanol > choloroform
(c) Acetic acid > choloroform > ethanol > methane
(d) Choloroform > ethanol > methane> acetic acid
Answer
Answer: (c)
Explanation:
Melting points and boiling points of some compounds of carbon :
Compound Melting point (K) Boiling point (K)
Acetic acid (CH3 COOH) 290 391
Chloroform (CHC13) 209 334
Ethanolji(CH3CH20H) 156 351
Methane (CH4) 90 111
2. Which of the following is/are the characteristics of parbon for the formation of large number of organic compounds?
1. Catenation
2. Tetravalency
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (c)
Explanation:
Carbon has the unique ability to form bonds with other atoms of carbon, giving rise to large molecules. This property is called catenation. These compounds may have long chains of carbon, branched chains of carbon or even carbon atoms arranged in rings. Since carbon has a valency of four, it is capable of bonding with four other atoms of carbon or atoms of some other monovalent element. Compounds of carbon are formed with oxygen, hydrogen, nitrogen, sulphur, chlorine and many other elements giving rise to compounds with specific properties which depend on the elements other than carbon present in the molecule.
3. Which of the following is/are correct for allotropes of carbon?
1. They are two in number.
2. In diamond, each carbon atom is bonded to four other carbon atoms.
3. In graphite, each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (b)
Explanation:
The element carbon occurs in different forms in nature with widely varying physical properties. Both diamond and graphite are formed by carbon atoms, the difference lies in the manner in which the carbon atoms are bonded to one another. In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid threedimensional structure. In graphite, each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array. Fullerenes form another class of carbon allot- ropes. The first one to be identified was C-60 which has carbon atoms arranged in the shape of a football.
4. Which of the following statements is/are correct for saturated compounds?
1. They are called alkanes.
2. They are single bonded.
3. They are very reactive.
(a) 1 and 2
(b) 1 and 3
(c) 2 and 3
(d) 1, 2 and 3
Answer
Answer: (a)
Explanation:
The valencies of all the atoms are satisfied by single bonds between them. Such carbon compounds are called saturated compounds. These compounds are normally not very reactive.
5. Which of the following is/are correct for homologous series?
1. The melting point increases as we go down in a series.
2. It is a series with different functional groups,
(a) Only 1
(b) Only 2
(c) Both 1 and 2
(d) Neither 1 nor 2
Answer
Answer: (a)
Explanation:
A homologous series is a series of compounds with the same functional group and similar chemical properties in which the members of the series can be branched or unbranched. As the molecular mass increases in any homologous series, a gradation in physical properties is seen. This is because the melting and boiling points increase with increasing molecular mass. Other physical properties such as solubility in a particular solvent also show a similar gradation. But the chemical properties, which are determined solely by the functional group, remain similar in a homologous series.
6. Which of the following are correctly matched?
1. Alkaline potassium permanganate _______ reducing agent
2. Addition reaction _______ hydrogenation of vegetable oil
3. Concentrated sulphuric acid _______ dehydrating agent.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1, 2 and 3
Answer
Answer: (b)
Explanation:
Alkaline potassium permanganate or acidified potassium dichromate are oxidising alcohols to acids, that is, adding oxygen to the starting material. Hence, they are known as oxidising agents. Addition reaction is commonly used in the hydrogenation of vegetable oils using a nickel catalyst. Vegetable oils generally have long unsaturated carbon chains while animal fats have saturated carbon chains. The concentrated sulphuric acid can be regarded as a dehydrating agent which removes water from ethanol.
7. Which of the following is/are true for ethanol?
1. Ethanol is an important industrial solvent.
2. Dyes added to alcohol is called as denatured alcohol.
3. It can be used as a fuel.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1,2 and 3
Answer
Answer: (a)
Explanation:
Ethanol is an important industrial solvent. To prevent the misuse of ethanol produced for industrial use, it is made unfit for drinking by add ing poisonous substances like methanol to it. Dyes are also added to colour the alcohol blue so that it can be identified easily. This is called denatured alcohol. Sugarcane juice can be used to prepare molasses which is fermented to give al-cohol (ethanol). Some countries now use alco hoi as an additive in petrol since it is a cleaner fuel which gives rise to only carbon dioxide and water on burning in sufficient air (oxygen).
Periodic Classification of Elements MCQ Questions
1. Which of the following statements is/are correct for dobereiner triads?
1. It was given in the year 1816.
2. It was a group of three elements.
3. When the three elements in a triad were written in the order of increasing atomic masses; the atomic mass of the middle element was roughly the average of the atomic masses of the other two elements.
(a) 1 and 2
(b) 2 and 3
(c) 1 and 3
(d) 1,2 and 3
Answer
Answer: (b)
Explanation:
In the year 1817, Johann Wolfgang Dobereiner, a German chemist, tried to arrange the elements with similar properties into groups. He identi- fled some groups having three elements each. So he called these groups triads’. Dobereiner showed that when the three elements in a triad were written in the order of increasing atomic masses; the atomic mass of the middle element was roughly the average of the atomic masses of the other two elements.
2. Which of the following statements is/are true for law of octaves.
1. It was applicable upto potassium.
2. Assumption was only 56 elements will exist.
3. It was given by John Newlands.
(a) Only 1
(b) Only 3
(c) 1 and 2
(d) 2 and 3
Answer
Answer: (d)
Explanation:
In 1866, John Newlands, an English scientist, arranged the then known elements in the order of increasing atomic masses. He started with the element having the lowest atomic mass (hydrogen) and ended at thorium which was the 56th element. He found that every eighth element had properties similar to that of the first. He compared this to the octaves found in music. It was found that the Law of Octaves was applicable only upto calcium, as after calcium every eighth element did not possess properties similar to that of the first. It was assumed by Newlands that only 56 elements existed in nature and no more elements would be discovered in the future.
3. Which of the following is not based on atomic mass?
1. Dobereiner triads
2. Newlands’ law of octaves
3. Mendeleev classification
4. Modem periodic table
(a) Only 1
(b) Only 2
(c) Only 3
(d) Only 4
Answer
Answer: (d)
Explanation:
Dobereiner showed that when the three elements in a triad were written in the order of increasing atomic masses; the atomic mass of the middle element was roughly the average of the atomic masses of the other two elements. In 1866, John Newlands, an English scientist, arranged the then known elements in the order of increasing atomic masses. He started with the element having the lowest atomic mass (hydrogen) and ended at thorium which was the 56th element. When Mendeleev started his work, 63 elements were known. He examined the relationship between the atomic masses of the elements and their physical and chemical properties. Mendeleev’s Periodic Law was modified and atomic number was adopted as the basis of Modem Periodic Table and the Modem Periodic Law can be stated as follows: ‘Properties of elements are a periodic function of their atomic number.
4. Which of the following statements is not a correct statement about the trends when going from left to right across the periods of periodic table.
(a) The elements become less metallic in nature.
(b) The number of valence electrons increases.
(c) The atoms lose their electrons more easily.
(d) The oxides become more acidic.
Answer
Answer: (c)
Explanation:
The valency of an element is determined by the number of valence electrons present in the outermost shell of its atom. As the atomic number increases from left to right in a period, therefore valence electrons also increases. The atomic radius decreases in moving from left to right along a period. This is due to an increase in nuclear charge which tends to pull the electrons closer to the nucleus and reduces the.size of the atom. Therefore, it becomes difficult to lose electrons. Hence, metallic character decreases across a pe-riod. Non-metals are found on the right-hand side of the Periodic Table towards the top. These trends also help us to predict the nature of oxides formed by the elements because it is known to you that the oxides of metals are basic and that of nonmetals are acidic in general.
