Free PDF Download of CBSE Chemistry Multiple Choice Questions for Class 12 with Answers Chapter 7 The p-Block Elements. Chemistry MCQs for Class 12 Chapter Wise with Answers PDF Download was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 12 Chemistry The p-Block Elements MCQs Pdf with Answers to know their preparation level.
The p-Block Elements Class 12 Chemistry MCQs Pdf
1. Among the following, which one is a wrong statement.
(a) PH5 and BiCl5 do not exist.
(b) pπ-dπ bonds are present in SO2
(c) SeF4 and CH4 have same shape.
(d) I3 has bent geometry.
Answer/Explanation
Answer: c
Explaination:
(c) SeF4 has see-saw shape where as CH4 is tetrahedral.
2. In which of the pair of ions, both species contain S—S bond?

Answer/Explanation
Answer:
Explaination:

3. Which one of the following order is correct for the bond dissociation enthalpy of halogen molecule?
(a) Br2 > I2 > F2 > Cl2
(b) F2 > Cl2 > Br2 > I2
(c) I2 > Br2 > Cl2 > F2
(d) Cl2 > Br2 > F2 > I2
Answer/Explanation
Answer: d
Explaination:
(d) In F2, therefore, inter electronic repulsion, therefore, bond dissociation enthalpy is less.
4. Which is strongest acid in the following:
(a) HClO4
(b) H2SO3
(c) H2SO4
(d) HClO3
Answer/Explanation
Answer: a
Explaination:
(a) HClO4 is strongest because ‘Cl’ has +7 oxidation state.
5. In which of the following pairs, the two species are isostructural

Answer/Explanation
Answer: c
Explaination:
(c) Both are pyramidal.

6. The correct order of oxidising power is
(a) HClO4 > HClO3 > HClO2 > HCIO
(b) HOCl > HClO2 > HClO3 > HClO4
(c) HClO3 > HClO4 > HClO2 > HClO
(d) HCIO2 > HOCl > HClO3 > HClO4
Answer/Explanation
Answer: b
Explaination:
(b) HOCl → HCl + [O]
It is strongest oxidising agent whereas HClO4 is weakest.
7. The correct order of acid strength is
(a) HClO4 < HClO3 < HClO2 < HClO
(b) HCIO < HClO2 < HClO3 < HClO4
(c) HClO4 < HClO < HClO2 < HClO3
(d) HClO2 < HClO3 < HClO4 < HClO
Answer/Explanation
Answer: b
Explaination:
(b) As oxidation state increases, acid strength increases.
8. Among the following which is strongest oxidising agent.
(a) Br2
(b) I2
(c) Cl2
(d) F2
Answer/Explanation
Answer: d
Explaination:
(d) F2 is best oxidising agent.
9. The correct order of bond angles in the following species is

Answer/Explanation
Answer: b
Explaination:
(b) ClO2– < Cl2O < ClO2 is increasing order of bond angle.
10. Sulphur trioxide can be obtained by which of the following:

Answer/Explanation
Answer:
Explaination:
![]()
11. When Cl2 reacts with hot and cone. NaOH, the oxidation number of chlorine changes from
(a) zero to +1 and zero to +5
(b) 0 to -1 and 0 to +5
(c) zero to -1 and zero to +3
(d) 0 to +1 and 0 to -3
Answer/Explanation
Answer: b
Explaination:
(b) Cl2 has oxidation number 0, in Cl (-1) and in ClO3(+5).
3Cl2 + 6NaOH(hot and cone.) → 5NaCl + NaClO3 + 3H2O
12. Acidity of diprotic acid in aqueous solution increases in the order.
(a) H2S < H2Se < H2Te
(b) H2Se < H2S < H2Te
(c) H2Te < H2S < H2Se
(d) H2Se < H2Te < H2S
Answer/Explanation
Answer: a
Explaination:
(a) Because bond dissociation enthalpy decreases as atomic size increases.
13. Which of the following statement is incorrect?
(a) ONF is isoelectronic with NO–2
(h) OF2 is an oxide of fluoride
(c) Cl2O7 is an anhydride of perchloric acid
(d) O3 molecule is bent
Answer/Explanation
Answer: b
Explaination:
(b) It is fluoride of oxygen.
∵ ‘F’ is more electronegative than O.
14. Chlorine reacts with excess of NH3 to form
(a) NH4Cl
(b) N2 + HCl
(c) N2 + NH4Cl
(d) NCl3 + HCl
Answer/Explanation
Answer: c
Explaination:
(c) 8NH3 + 3Cl2 → 6NH4Cl + N2
15. Which of the following reactions is an example of redox reaction?
(a) XeF4 + O2F2 → XeF6 + O2
(b) XeF2 + PF5 → [XeF]+ [PF6]–
(c) XeF6 + H2O → XeOF4 + 2HF
(d) XeF6 + 2H2O → Xeo2F2 + 2HF
Answer/Explanation
Answer: a
Explaination:
(a) Redox reaction becaueXe(+4) is getting oxidised to Xe(+6) and 0(+l) is reduced to zero.
16. On addition of cone. H2SO4 to a chloride salt, colourless fumes are evolved but in case of iodide salt, violet flames come out. This is because [NCERT Exemplar]
(a) H2SO4 reduces HI to I2
(b) HI is of violet colour
(c) HI gets oxidised to I2
(d) HI changes to HIO3
Answer/Explanation
Answer: c
Explaination:
(c) HI gets oxidised to I2
17. Which of the following pairs of ions are isoelectronic and isostructural? [NCERT Exemplar]

Answer/Explanation
Answer: a
Explaination:
(a) CO–3 and NO–3 are isoelectronic (32 electrons) and Planar.

18. Affinity for hydrogen decreases in the group from fluorine to iodine. Which of the halogen acids should have highest bond dissociation enthalpy? [NCERT Exemplar]
(a) HF
(b) HCl
(c) HBr
(d) HI
Answer/Explanation
Answer: a
Explaination:
(a) HF has highest bond dissociation enthalpy due to smaller bond length.
19. Bond dissociation enthalpy of E—H (E = element) bonds is given below. Which of the compounds will act as strongest reducing agent? [NCERT Exemplar]

(a) NH3
(b) PH3
(c) AsH3
(d) SbH3
Answer/Explanation
Answer: d
Explaination:
(d) SbH3 due to lowest bond dissociation enthalpy.
20. Hot cone. H2SO4 acts as moderately strong oxidising agent. It oxidises both metals and non-metals. Which of the following element is oxidised by cone. H2SO4 into two gaseous products? [NCERT Exemplar]
(a) Cu
(b) S
(c) C
(d) Zn
Answer/Explanation
Answer: c
Explaination:
(c) C + 2H2SO4(conc.) → CO2 + SO2 + 2H2O
21. H2S is more acidic than H2O because
(a) oxygen is more electronegative than sulphur.
(b) atomic number of sulphur is higher than oxygen.
(c) H — S bond dissociation energy is less as compared to H — O bond.
(d) H — O bond dissociation energy is less also compared to H — S bond.
Answer
Answer: b
22. The boiling points of hydrides of group 16 are in the order
(a) H2O > H2Te > H2S > H2Se
(b) H2O > H2S > H2Se > H2Te
(c) H2O > H2Te > H2Se > H2S
(d) None of these
Answer
Answer: b
23. In the manufacture of sulphuric acid by contact process Tyndall box is used to
(a) convert SO2 and SO3
(b) test the presence of dust particles
(c) filter dust particles
(d) remove impurities
Answer
Answer: b
24. Fluorine differs from rest of the halogens in some of its properties. This is due to
(a) its smaller size and high electronegativity.
(b) lack of d-orbitals.
(c) low bond dissociation energy.
(d) All of the these.
Answer
Answer: b
25. The set with correct order of acidity is
(a) HClO < HClO2 < HClO3 < HClO4
(b) HClO4 < HClO3 < HClO2 < HClO
(c) HClO < HClO4 < HClO3 < HClO2
(d) HClO4 < HClO2 < HClO3 < HClO
Answer
Answer: b
26. When chlorine reacts with cold and dilute solution of sodium hydroxide, it forms
(a) Cl– and ClO–
(b) Cl– and ClO2–
(c) Cl– and ClO3–
(d) Cl– and ClO4–
Answer
Answer: a
27. The formation of O2+ [PtF6]– is the basis for the formation of first xenon compound. This is because
(a) O2 and Xe have different sizes.
(b) both O2 and Xe are gases.
(c) O2 and Xe have comparable electro-negativities.
(d) O2 and Xe have comparable ionisation enthalpies.
Answer
Answer: d
28. Partial hydrolysis of XeF4 gives
(a) XeO3
(b) XeOF2
(c) XeOF4
(d) XeF2
Answer
Answer: b
29. Helium is preferred to be used in balloons instead of hydrogen because it is
(a) incombustible
(b) lighter than hydrogen
(c) more abundant than hydrogen
(d) non polarizable
Answer
Answer: a
30. The increasing order of reducing power of the halogen acids is
(a) HF < HCl < HBr < HI
(b) HI < HBr < HCl < HF
(c) HBr < HCl < HF < HI
(d) HCl < HBr < HF < HI
Answer
Answer: a
Note: In the following questions two or more options may be correct. (Q 21. to Q 24)
31. Which of the following options are not in accordance with the property mentioned against them? [NCERT Exemplar]
(a) F2 > Cl2 > Br2 > I2 Oxidising power.
(b) MI > MBr > MCI > MF Ionic character of metal halide.
(c) F2 > Cl2 >Br2 > I2 Bond dissociation enthalpy.
(d) HI < HBr < HCl < HF Hydrogen-halogen bond strength.
Answer/Explanation
Answer:
Explaination:
(b) MF > MCl > HBr > MI Ionic character
(c) Cl2 > Br2 > F2 > I2
32. Which of the following statements are correct? [NCERT Exemplar]
(a) Among halogens, radius ratio between iodine and fluorine is maximum.
(b) Leaving F—F bond, all halogens have weaker X—X bond than X—X’ bond in interhalogens.
(c) Among interhalogen compounds maximum number of atoms are present in iodine fluoride.
(d) Interhalogen compounds are more reactive than halogen compounds.
Answer/Explanation
Answer:
Explaination:
(a), (c) and (d) are correct.
F2 is more reactive than interhalogen compounds.
(b) is not correct, other halogens are less reactive than interhalogen compounds
33. Which of the following statements are correct for SO2 gas? [NCERT Exemplar]
(a) It acts as bleaching agent in moist conditions.
(b) It’s molecule has linear geometry.
(c) It’s dilute solution is used as disinfectant.
(d) It can be prepared by the reaction of dilute H2S04 with metal sulphide.
Answer/Explanation
Answer:
Explaination:
(b) and (d). Its molecule is bent.

and Na2S + H2SO4 → Na2SO4 + H2S
34. Which of the following orders are correct as per the properties mentioned against each? [NCERT Exemplar]
(a) AS2O3 < SiO2 < P2O3 < SO2 Acid strength.
(b) AsH3 < PH3 < NH3 Enthalpy of vapourisation,
(c) S < O < Cl < F More negative electron gain enthalpy.
(d) H2O > H2S > H2Se > H2Te Thermal stability.
Answer/Explanation
Answer:
Explaination:
(a) and (d) are correct (b) and (c) are wrong.
(b) PH3 <ASH3 <NH3
(c) Cl > F > S > O
35. Match the compounds given in Column I with the hybridisation and shape given in Column II and mark the correct option. [NCERT Exemplar]
| Column I | Column II |
| (A) Xe F6 | (1) sp3d3 – distorted octahedral |
| (B) Xe O3 | (2) sp3d2 – square planar |
| (C) Xe OF4 | (3) sp3– pyramidal |
| (D) Xe F4 | (4) sp3 d2 – square pyramidal |
Code:
(a) A (1) B (3) C (4) D (2)
(b) A (1) B (2) C (4) D (3)
(c) A (4) B (3) C (1) D (2)
(d) A (4) B (1) C (2) D (3)
Answer/Explanation
Answer:
Explaination:
(a) A (1) B (3) C (4) D (2)
36. Match the items of Columns I and II and mark the correct option. [NCERT Exemplar]
| Column I | Column II |
| (A) H2SO4 | (1) Highest electron gain enthalpy |
| (B) CCl3NO2 | (2) Chalcogen |
| (C) Cl2 | (3) Tear gas |
| (D) Sulphur | (4) Storage batteries |
Answer/ExplanationCode:
(a) A (4) B (3) C (1) D (2)
(b) A (3) B (4) C (1) D (2)
(c) A (4) B (1) C (2) D (3)
(d) A (2) B (1) C (3) D (4)
Explaination:
(a) A (4) B (3) C (1) D (2)
Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (Q.27 to Q.29)
(a) Both assertion and reason are correct statements, and reason is the correct explanation of the assertion.
(b) Both assertion and reason are correct statements, but reason is not the correct explanation of the assertion.
(c) Assertion is correct, but reason is wrong statement.
(d) Assertion is wrong but reason is correct statement.
(e) Both assertion and reason are wrong statements.
37. Assertion: HI cannot be prepared by the reaction of KI with concentrated H2SO4.
Reason: HI has lowest H-X bond strength among halogen acids.[NCERT Exemplar]
Answer/Explanation
Answer: b
Explaination:
(b) Both assertion and reason are correct statements, but reason is not the correct explanation of the assertion. HI gets oxidised to I2 as H2SO4(conc.) is oxidising agent.
38. Assertion: Both rhombic and monoclinic sulphur exist as S8 but oxygen exists as O2.
Reason: Oxygen forms pπ – pπ multiple bond due to small size and small bond length but pπ – pπ bonding is not possible in sulphur. [NCERT Exemplar]
Answer/Explanation
Answer: a
Explaination:
(a) Both assertion and reason are correct statements, and reason is the correct explanation of the assertion.
39. Assertion: NaCl reacts with concentrated H2SO4 to give colourless fumes with pungent smell. But on adding MnO2 the fumes become greenish yellow.
Reason: Mn02 oxidises HC1 to chlorine gas which is greenish yellow. [NCERT Exemplar]
Answer/Explanation
Answer: a
Explaination:
(a) Both assertion and reason are correct statements, and reason is the correct explanation of the assertion.
NaCl + H2SO4(conc.) → NaHSO4 + HCl
4HCl + MnO2 → MnCl2 + Cl2 + 2H2O
40. The mixture of cone. HCl and anhydrous ZnCl2 is called ___________ .
Answer/Explanation
Answer:
Explaination: Lucas reagent
41. Out of H2O and H2S which has higher bond angle? ___________ .
Answer/Explanation
Answer:
Explaination: H20
42. Tin reacts with excess of chlorine gas to form ___________ .
Answer/Explanation
Answer:
Explaination: SnCl4
43. Lead sulphide is heated with air to form ___________ .
Answer/Explanation
Answer:
Explaination: PbO + SO2
44. I22 gets oxidised to ___________ by cone. HNO3.
Answer/Explanation
Answer:
Explaination: HIO3
45. Interhalogen compounds are more reactive than helogens except fluorine. [True/False]
Answer/Explanation
Answer:
Explaination: True. It is due to less effective overlapping.
46. C1F is neutral molecule isoelectronic withCIO–. [True/False]
Answer/Explanation
Answer:
Explaination: True. Both have 17 + 9 = 26 electrons.
47. NaF reacts with SbF6 to form Na+ [SbF7]–. [True/False]
Answer/Explanation
Answer:
Explaination:
![]()
48. Hydrolysis of XeF6 is redox reaction. [True/False]
Answer/Explanation
Answer:
Explaination: It is false. XeF6 + 3H2O > XeO3 + 6HF.
49. Ozone is thermodynamically less stable than O2. [True/False]
Answer/Explanation
Answer:
Explaination: True
50. Oxygen does not show +4 and +6 oxidation states like sulphur. Why?
Answer/Explanation
Answer:
Explaination: It is due to the absence of vacant d-orbital in oxygen.
51. Draw the structure of 03 molecule.
Answer/Explanation
Answer:
Explaination:

It is bent molecule.
52. Write one chemical reaction equation to show that SO2 acts as a reducing agent.
Answer/Explanation
Answer:
Explaination:
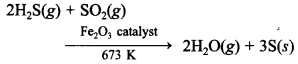
53. State reason for the following:
Sulphur has greater tendency for catenation than oxygen. [Delhi 2013; AI2016,12; DoE]
Or
O-O bond is weaker than S-S bond, why? [Chennai 2019]
Answer/Explanation
Answer:
Explaination:
It is because of strong S—S bond than 0—0 due to greater repulsion between valence electrons of smaller atoms of oxygen than sulphur atoms.
54. Write the formulae of any two oxoacids of sulphur. [AI 2015 Allahabad & Dehradun]
Answer/Explanation
Answer:
Explaination:
H2SO4 and H2SO3.
55. Write one chemical reaction equation to show that cone. H2S04 is a strong oxidising agent.
Answer/Explanation
Answer:
Explaination:
C + 2H2SO4(conc.) CO2+ 2H2O + 2SO2
56. Fluorine does not exhibit any positive oxidation state. Why? [Delhi 2013]
Answer/Explanation
Answer:
Explaination:
It is because Fluorine is most electronegative element and best oxidising agent.
57. Despite lower value of its electron gain enthalpy with negative sign, fluorine (F2) is a stronger oxidising agent than Cl2. [Delhi 2019; AI 2012; DoE]
Answer/Explanation
Answer:
Explaination:
It is due to higher standard reduction potential of F2 which is due to low bond dissociation energy of F—F bond due to inter electronic repulsion among small size F atoms, high electron gain enthalpy and highest hydration enthalpy.
58. Although the H-bonding in hydrogen fluoride is much stronger than that in water, yet water has a much higher boiling point than hydrogen fluoride. Why? [Foreign 2012]
Answer/Explanation
Answer:
Explaination:
It is because H20 molecules form H-bonds to more extent as compared to HF molecules.
59. Give a chemical reaction involved in chlorine with saturated hydrocarbon.
Answer/Explanation
Answer:
Explaination:

60. Explain giving a reason for the following situation:
In aqueous medium, HCl is a stronger acid than HF. [Foreign 2011]
Answer/Explanation
Answer:
Explaination:
It is because bond dissociation energy of H—Cl is lower than HF due to longer bond length.
61. Give the chemical formula of any one halic acid.
Answer/Explanation
Answer:
Explaination: H0BrO2 (Bromic acid)
62. Give a chemical reaction involved in the preparation of interhalogen compound.
Answer/Explanation
Answer:
Explaination:
![]()
63. Which compound led to the discovery of the compounds of noble gas?
Answer/Explanation
Answer:
Explaination:
which was the first compound of noble gas.
64. Complete the following reaction:
Xe + PtF6 →
Answer/Explanation
Answer:
Explaination:
Xe + PtF6 → Xe+[PtF6]–
65. Sulphur disappears when boiled with an aqueous solution of sodium sulphite. Why?
Answer/Explanation
Answer:
Explaination:
It is due to the formation of sodium thiosulphate which is soluble in water, therefore, sulphur disappears.
![]()
We hope the given Chemistry MCQs for Class 12 with Answers Chapter 7 The p-Block Elements will help you. If you have any query regarding CBSE Class 12 Chemistry The p-Block Elements MCQs Pdf, drop a comment below and we will get back to you at the earliest.