Free PDF Download of CBSE Chemistry Multiple Choice Questions for Class 12 with Answers Chapter 4 Chemical Kinetics. Chemistry MCQs for Class 12 Chapter Wise with Answers PDF Download was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 12 Chemistry Chemical Kinetics MCQs Pdf with Answers to know their preparation level.
Chemical Kinetics Class 12 Chemistry MCQs Pdf
1. The half life period of first order reaction is 1386 seconds. The specific rate constant of the reaction is
(a) 0.5 × 10-2 s-1
(b) 0.5 × 10-3 s-1
(c) 5.0 × 10-2 s-1
(d) 5.0 × 10-3 s-1
Answer/Explanation
Answer: b
Explaination:
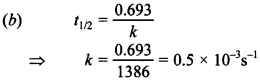
2. The rate constant of a reaction A → B is 0.6 × 103 mole per second. If the concentration of [A] is 5 M, then what will be concentration of [B] after 20 months?
(a) 0.36 M
(b) 0.72 M
(c) 1.08 M
(d) 3.60 M
Answer/Explanation
Answer: b
Explaination:

3. Which of the following is affected by catalyst?
(a) ∆H
(b) ∆S
(c) ∆G
(d) Ea
Answer/Explanation
Answer: d
Explaination:
(d) Catalyst affects activation energy. It does not affect, ∆H, ∆S, ∆G.
4. A first order reaction has specific reaction rate 10-2s-1. How much time it will take for 20g of reactant to reduce to 5g?
(a) 138.6 s
(b) 346.5 s
(c) 693.0 s
(d) 238.6 s
Answer/Explanation
Answer: a
Explaination:
![]()
5. The activation energy of a reaction can be determined from the slope of which of the following graph:
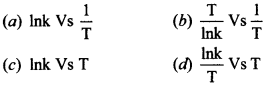
Answer/Explanation
Answer: a
Explaination:
(a) When we plot Ink Vs \(\frac{1}{T}\)
slope of line = \(-\frac{E_{a}}{R}\)
6. Mechanism of a hypothetical reaction X2 + Y2 → 2 × Y is given below:
![]()
(b) X + Y2 → XY + Y (slow)
(c) X + Y → XY (fast)
The overall order of reaction is
(a) 2
(b) 0
(c) 1.5
(d) 1
Answer/Explanation
Answer: c
Explaination:
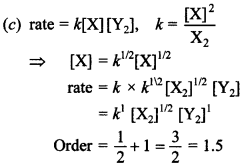
7. The rate of first order reaction is 0.04 mol L-1 s-1 at 10 sec. and 0.03 mol L-1 at 2C seconds after initiation of the reaction. t1/2 of reaction is
(a) 44.1 s
(b) 54.1 s
(c) 24.1 s
(d) 34.1 s
Answer/Explanation
Answer: c
Explaination:
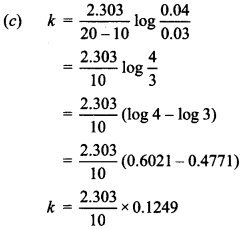
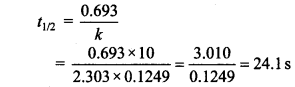
8. If the initial concentration of reactant is doubled, t1/2 is also doubled, the order of reaction is
(a) zero
(b) 1
(c) 2
(d) 3
Answer/Explanation
Answer: a
Explaination:
(a) In zero order of reaction t1/2 ∝ [R]0
9. For a reaction taking place in three steps,

(a) 30
(b) 40
(c) 60
(d) 50
Answer/Explanation
Answer: a
Explaination:
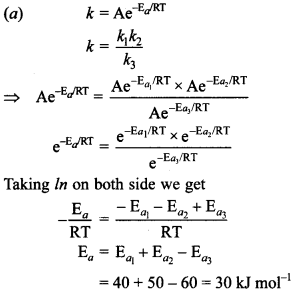
10. If cone, of reactant ‘A’ is increased 10 times and rate of reaction becomes 100 times. What is order with respect to ‘A’?
(a) 1
(b) 1
(c) 3
(d) 4
Answer/Explanation
Answer: b
Explaination:
(b) rate = k[A]x
100 x rate = k[10]x
⇒ x = 2.
11. In the first order reaction the concentration of reactant decreases from 0.6 M to 0.3 M in 30 minutes. The time taken for the concentration to change from 0.1 M to 0.025 M:
(a) 60 min
(b) 30 min
(c) 15 min
(d) 50 min
Answer/Explanation
Answer: a
Explaination:
(a) t3/4= 2t1/2 = 2 × 30 = 60 min.
| 0.6 M to 0.3 M means t1/2 = 30 min ∴ 0.1 M to 0.025 M, concentration has become 1/4 |
12. Consider Fig. and mark the correct option. [NCERT Exemplar]
(а) Activation energy of forward read ion is E1 + E2 and product is less stable than reactant.
(b) Activation energy of forward reaction is E1 + E2 and product is more stable than reactant.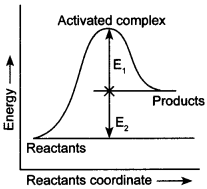
(c) Activation energy of both forward and backward reaction is E1 + E2 and reactant is more stable than product.
(d) Activation energy of backward reaction is E1 and product is more stable than reactant.
Answer/Explanation
Answer: a
Explaination:
(a) Ea = E1 + E2 and products are less stable due to higher energy.
13. Consider the Arrhenius equation given below and mark the correct option. [NCERT Exemplar]
k = A \(\mathrm{e}^{-\mathrm{E}_{\mathrm{a}} / \mathrm{RT}}\)
(a) Rate constant increases exponentially with increasing activation energy and decreasing temperature.
(b) Rate constant decreases exponentially with increasing activation energy and decreasing temperature.
(c) Rate constant increases exponentially with decreasing activation energy and decreasing temperature.
(d) Rate constant increases exponentially with decreasing activation energy and increasing temperature.
Answer/Explanation
Answer: d
Explaination:
(d) Rate constant (k) increases exponentially by decreasing activation energy and increasing temperature because lesser the activation energy and temperature, more will be rate of reaction and rate constant ‘k’
14. A first order reaction is 50% completed in 1.26 × 1014 s. How much time would it take for 100% completion? [NCERT Exemplar]
(a) 1.26 × 1015 s
(b) 2.52 × 1014 s
(c) 2.52 × 1028 s
(d) infinite
Answer/Explanation
Answer: d
Explaination:
(d) First order reaction is never 100% complete,
therefore, t100% = infinity.
15. Activation energy of a chemical reaction can be determined by __________ . [NCERT Exemplar]
(a) determining the rate constant at standard temperature.
(b) determining the rate constants at two temperatures.
(c) determining probability of collision.
(d) using catalyst.
Answer/Explanation
Answer: b
Explaination:
![]()
By knowing kx at T, and k2 at T2, we can determine Ea (activation energy)
16. For the reaction N2 + 3H2 → 2NH3 if \(\frac{\Delta\left[\mathrm{NH}_{3}\right]}{\Delta t}\) = 2 × 10-4 mol L-1s-1, the value of \(\frac{-\Delta\left[\mathrm{H}_{2}\right]}{\Delta t}\) would be
(a) 1 × 10-4 mol L-1s-1
(b) 3 × 10-4 mol L-1s-1
(c) 4 × 10-4 mol L-1s-1
(d) 6 × 10-4 mol L-1s-1
Answer
Answer: b
17. The rate of a certain hypothetical reaction
A + B + C → products
is given by r = \(\frac{-d[\mathbf{A}]}{d t} \mathbf{K}[\mathbf{A}]^{1 / 2}[\mathbf{B}]^{1 / 3}[\mathbf{C}]^{1 / 4}\). The order of the reaction is
(a) 13/11
(b) 13/14
(c) 12/13
(d) 13/12
Answer
Answer: d
18. In the formation of S02 by contact process;
2SO2 + O2 → 2SO3, the rate of reaction was measured as \(\frac{-d\left[\mathrm{O}_{2}\right]}{d t}\) = 2.5 × 10-4 mol L-1s-1. at
The rate of formation of of S03 will be
(a) -5.0 × 10-4 mol L-1s-1
(b) -1.25 × 10-4 mol L-1s-1
(c) 3.75 × 10-4 mol L-1s-1
(d) 5.00 × 10-4 mol L-1s-1
Answer
Answer: d
19. For a chemical reaction A→B, it is found that the rate of reaction doubles when the concentration of A is increased four times. The order of reaction is
(a) Two
(b) One
(c) Half
(d) Zero
Answer
Answer: c
20. The half life of the first order reaction having rate constant K = 1.7 x 10-5s-1 is
(a) 12.1 h
(b) 9.7 h
(c) 11.3 h
(d) 1.8 h
Answer
Answer: c
21. What will be the fraction of molecules having energy equal to or greater than activation energy, Ea?
(a) K
(b) A
(c) Ae-Ea/Rt
(d) e-Ea/Rt
Answer
Answer: d
22. ![]()
What type of reaction is this?
(a) Second order
(b) Unimolecular
(c) Pseudo-unimolecular
(d) Third order
Answer
Answer: c
23. Which among the following is a false statement?
(a) Rate of zero order reaction is independent of initial concentration of reactant.
(b) Half life of a third order reaction is inversely proportional to square of initial concentration of the reactant.
(c) Molecularity of a reaction may be zero or fraction.
(d) For a first order reaction, \(t_{1 / 2}=\frac{0.693}{\mathrm{K}}\)
Answer
Answer: c
24. Which of the following statements about the catalyst is true?
(a) A catalyst accelerates the rate of reaction by bringing down the activation energy.
(b) A catalyst does not participate in reaction mechanism.
(c) A catalyst makes the reaction feasible by making ∆G more negative.
(d) A catalyst makes equilibrium constant more favourable for forward reaction.
Answer
Answer: a
25. An endothermic reaction with high activation energy for the forward reaction is given by the diagram.
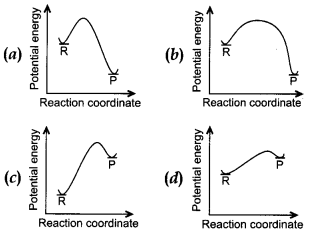
Answer
Answer: c
Note: In the following questions two or more options may be correct. (Q.16 to Q.19)
26. Rate law cannot be determined from balanced chemical equation if __________ . [NCERT Exemplar]
(a) reverse reaction is involved.
(b) it is an elementary reaction.
(c) it is a sequence of elementary reactions.
(d) any of the reactants is in excess.
Answer/Explanation
Answer:
Explaination:
(a), (c) and (d). Rate law can be determined by balanced chemical equation if it is elementary reaction.
27. Which of the following statements are applicable to a balanced chemical equation of an elementary reaction? [NCERT Exemplar]
(a) Order is same as molecularity.
(b) Order is less than the molecularity.
(c) Order is greater than the molecularity.
(d) Molecularity can never be zero.
Answer/Explanation
Answer:
Explaination:
(a) and (d), order and molecularity are equal in elementary reaction. Order can be zero but molecularity can never be zero.
28. At high pressure the following reaction is zero order.
![]()
Which of the following options are correct for this reaction? [NCERT Exemplar]
(a) Rate of reaction = Rate constant
(b) Rate of the reaction depends on concentration of ammonia.
(c) Rate of decomposition of ammonia will remain constant until ammonia disappears completely.
(d) Further increase in pressure will change the rate of reaction.
Answer/Explanation
Answer: a
Explaination:
(a), (c) and (d) In zero order reaction k = rate of reaction.
• Rate of reaction does not depend on concentration of reactants in zero order.
• Further increase in pressure will increase the rate of reaction because more number of collisions will take place.
29. According to Maxwell Boltzmann distributon of energy, _________ . [NCERT Exemplar]
(a) the fraction of molecules with most probable kinetic energy decreases at higher temperatures.
(b) the fraction of molecules with most probable kinetic energy increases at higher temperatures.
(c) most probable kinetic energy increases at higher temperatures.
(d) most probable kinetic energy decreases at higher temperatures.
Answer/Explanation
Answer:
Explaination:
(a) and (d). Number of molecules possessing higher kinetic energy increases with increase in temperature but most probable kinetic energy and fraction of molecules with it decreases because they acquire more kinetic energy and shift to right side.
30. Match the graph given in Column I with the order of reaction given in Column II.
More than one item in Column I may link to the same item of Column II. [NCERT Exemplar]
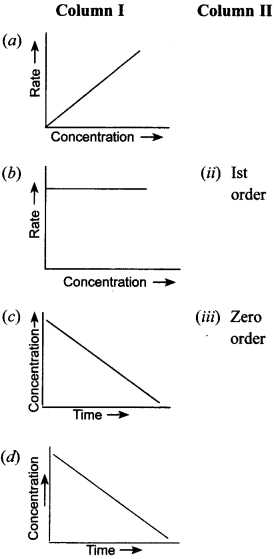
Answer/Explanation
Answer:
Explaination:
(a) and (d) are for 1st order.
∵ Rate ∝ [cone.] and log [cone.] decreases with time with negative slope.
(a) and (c) are of zero order.
∴ rate ∝ [conc]°, cone, decreases with time.
31. MatchthestatementsgiveninColumnland Column II. [NCERT Exemplar]
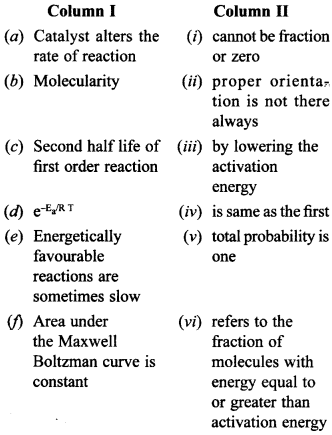
Answer/Explanation
Answer:
Explaination:
(a) (iii) Catalyst alters the rate of reaction by lowering the activation energy.
(b) (i) Molecularity cannot be fraction or zero.
(c) (iv) Second half life of first order reaction is same as the first.
(d) (vi) \(\mathrm{e}^{-\mathrm{E}_{\mathrm{a}} / \mathrm{R} \mathrm{T}}\) refers to the fraction of molecules with energy equal to or greater than activation energy
(e) (ii) Energetically favourable reactions are sometimes slow, proper orientation is not there always.
(f) (v) Area under the Maxwell Boltzman curve is constant and total probability is one.
Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (Q.22 and Q.23)
(a) Both assertion and reason are correct and the reason is correct explanation of assertion.
(b) Both assertion and reason are correct but reason does not explain assertion.
(c) Assertion is correct but reason is incorrect.
(d) Both assertion and reason are incorrect.
(e) Assertion is incorrect but reason is correct.
32. Assertion: Order of the reaction can be zero or fractional.
Reason: We cannot determine order from balanced chemical equation. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(b) Both assertion and reason are correct but reason does not explain assertion.
33. Assertion: Order and molecularity are same.
Reason: Order is determined experimentally and molecularity is the sum of the stoichiometric coefficient of rate determining elementary step. [NCERT Exemplar]
Answer/Explanation
Answer: e
Explaination:
(e) Assertion is incorrect but reason is correct. Order and molecularity may or may not be same.
34. In Bimolecular reaction if one of the reactants is in excess it is called _________ .
Answer/Explanation
Answer:
Explaination: Pseudo first order
35. If activation energy for forward reaction (Ea)b = 150 kJ and (Ea)b is 260 kJ, ∆H of reaction
Answer/Explanation
Answer:
Explaination:
∆H = [Ea]f – [Ea]b = 150 – 260
= -110 kJ mol-1
36. If t of first order and zero order are same. Then the ratio of the initial rates of first order reaction to the zero order reaction is ______________ .
Answer/Explanation
Answer:
Explaination: 2 × 0.693
37. SN1 (substitution nucleophilic reaction) in Tertiary halide is bimolecular but order is 1. [True/False]
Answer/Explanation
Answer:
Explaination: True
38. SN2 (substitution nucleophilic reaction) in Primary halide is bimolecular and order is 2. [True/False]
Answer/Explanation
Answer:
Explaination: True
39. t99% = 2t90%, in first order reaction. [True/False]
Answer/Explanation
Answer:
Explaination: True
40. t1/2 is inversely proportional to initial concentration in zero order reaction. [True/False]
Answer/Explanation
Answer:
Explaination:
False, t1/2 is directly proportional to initial cone, in zero order.
41. Why does the rate of a reaction not remain constant throughout the reaction process?
Answer/Explanation
Answer:
Explaination:
It is because the concentration of reactants goes on decreasing with time.
42. Express the rate of the following reaction in “terms of disappearance of hydrogen in the reaction: 3H2 (g) + N2 (g) → 2NH3 (g).
Answer/Explanation
Answer:
Explaination:
\(-\frac{1}{3} \frac{d\left[\mathrm{H}_{2}\right]}{d t}\) represents rate of reaction.
43. What will be the units of rate of a reaction in gaseous reactions?
Answer/Explanation
Answer:
Explaination:
When the concentration of gases is represented in terms of their partial pressure then the units of the rate of a reaction will be atm s-1.
44. What is the difference between law of mass action and rate law?
Answer/Explanation
Answer:
Explaination:
The law of mass action is an theoretical law and it is based on the balanced chemical reaction whereas rate law is an experimental law.
45. For a reaction, , A → B, the rate of reaction can be denoted by \(-\frac{d[A]}{d t} \text { or }+\frac{d[B]}{d t}\). State the significance of plus and minus sign.
Answer/Explanation
Answer:
Explaination:
Minus sign indicates the decrease in the concentration of reactant A with time whereas plus sign indicates the increase in the concentration of product B with time.
46. What is meant by order of a reaction being zero?
Answer/Explanation
Answer:
Explaination:
It means the reaction is extremely fast and the rate of reaction does not vary with the concentration of the reactants as it gets completed within seconds.
47. The graph given below is a plot of the rate of a reaction vs concentration of the reactant. What is the order of the reaction?
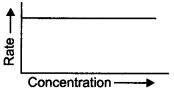
Answer/Explanation
Answer:
Explaination: Zero order reaction.
48. The graph given below is a plot of the rate of reaction vs concentration of the reactant. What is the order of the reaction?
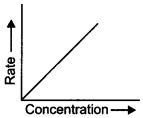
Answer/Explanation
Answer:
Explaination: First order reaction.
49. State a condition in which bimolecular reaction is kinetically first order reaction.
Answer/Explanation
Answer:
Explaination:
When one of the reactants is in excess, bimolecular reaction becomes kinetically first order.
50. Identify the reaction order from each of the following rate constants:
(i) k = 2.3 × 10-5 L mol-1 s-1
(ii) k = 3 × 10-4 s-1
Answer/Explanation
Answer:
Explaination:
(i) The unit of second order reaction is L mol-1 s-1 therefore, it represents second order reaction.
(ii) The unit of first order reaction is s-1, therefore, it is a first order reaction.
51. Calculate the overall order of a reaction which has the rate expression
(a) Rate = k [A]1/2 [B]3/2
(b) Rate = k [A]3/2 [B]-1 [Delhi 2011(C); AI 2011(C)]
Answer/Explanation
Answer:
Explaination:
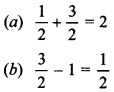
52. For the reaction Cl2(g)+2NO(g) → 2NOCl(g), the rate law is expressed as rate = k[Cl2][NO]2
What is the overall order of this reaction?
Answer/Explanation
Answer:
Explaination:
The overall order of reaction is 3.
53. For which type of reactions, order and molecularity have same value?
Answer/Explanation
Answer:
Explaination:
If the reaction is elementary reaction, order and molecularity are equal.
54. Why is the probability of reaction with molecularity higher than three very rare?
Answer/Explanation
Answer:
Explaination:
It is because multimolecular collisions have least or rare probability.
55. For a hypothetical reaction A+ 2B → C, it is found that the rate = k [A] [B]. What is the most likely rate determining step?
Answer/Explanation
Answer:
Explaination:
A + B → C is most likely to be rate determining step.
56. What is the shape of graph between log k vs \(\frac{1}{T}\)?
What is the relationship between its slope and activation energy (Ea)?
Answer/Explanation
Answer:
Explaination:
It is a straight line whose slope is – \(\frac{E_{a}}{2.303 R}\).
57. Express the relation between the half-life period of a reactant and its initial concentration for a reaction of nth order.
Answer/Explanation
Answer:
Explaination:
t1/2 ∝ \(\frac{1}{[R]_{0}^{n-1}}\) where is the order of reaction.
58. In the Arrhenius equation, what does the factor e-Ea/RT corresponds to? [CBSE Sample Paper 2017]
Answer/Explanation
Answer:
Explaination:
It represents fraction of molecules possessing energy equal to or more than activation energy.
59. According to Arrhenius, rate of reaction increases with increase in temperature. Give reasons.
Answer/Explanation
Answer:
Explaination:
Rate of a reaction increases with increase in temperature because when kinetic energy of molecules increases, the number of molecules possessing activation energy also increases, i.e. number of effective collisions increases and therefore, the rate of reaction increases.
50. Define threshold energy of a reaction.
Answer/Explanation
Answer:
Explaination:
Threshold energy is the minimum energy which must be possessed by reacting molecules in order to undergo effective collision which leads to formation of product molecule.
We hope the given Chemistry MCQs for Class 12 with Answers Chapter 4 Chemical Kinetics will help you. If you have any query regarding CBSE Class 12 Chemistry Chemical Kinetics MCQs Pdf, drop a comment below and we will get back to you at the earliest.