Free PDF Download of CBSE Chemistry MCQs for Class 12 with Answers Chapter 10 Haloalkanes and Haloarenes. Chemistry MCQs for Class 12 Chapter Wise with Answers was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 12 Chemistry Haloalkanes and Haloarenes MCQs Pdf with Answers to know their preparation level.
Haloalkanes and Haloarenes Class 12 Chemistry MCQs Pdf
1. Which of the following undergoes nucleophilic substitution exclusively by SN 1 mechanism?
(a) Benzyl chloride
(b) Ethyl chloride
(c) Chlorobenzene
(d) Isopropyl chloride
Answer/Explanation
Answer: a
Explaination:
(a) It is because benzyl carbocation is stabilised by resonance.
2. The increasing order of nucleophilicity would be
(a) Cl– < Br– < I–
(b) I– < Cl– < Br–
(c) Br– < Cl– < F–
(d) I– < Br– < Cl–
Answer/Explanation
Answer: a
Explaination:
(a) l– is best nucleophile because it can donate electron easily.
3.

Answer/Explanation
Answer: a
Explaination:
(a) ∵ 3° carbocation is most stable.

4. Which of the following is most reactive towards SN1 reaction?
(a) C6H5C(CH3)C6H5Br
(b) C6H5CH2Br
(c) C6H5CH(C6H5)Br
(d) C6H5CH(CH3)Br
Answer/Explanation
Answer: a
Explaination:
(a) Since it is 3° halide, carbocation is stabilised by resonance.
5. The correct order of increasing the reactivity of C—X bond towards nucleophile in following compounds

(a) IV < III < I < II
(c) I < II < IV < III
(b) III < II < I < IV
(d) II < III < I < IV
Answer/Explanation
Answer: c
Explaination:
(c) III is most reactive due to stability of 3° carbocation, -NO2 (electron withdrawing) group increase nucleophilic substitution reaction.
6. m-Xylene reacts with Br2 in presence of FeBr3, what are products formed


Answer/Explanation
Answer: c
Explaination:
(c) It is due to stearic hinderance, these products will be formed, —CH3 group is o andp-directing.
7. Which of the following compound will undergo racemisation when reacts with aq. KOH?

(a) (i) and (ii)
(b) (ii) and (iv)
(c) (iii) and (iv)
(d) (iv)
Answer/Explanation
Answer: d
Explaination:
(d) IV.
Since, it has chiral ‘C’ atom, others do not have.
8.

Answer/Explanation
Answer: c
Explaination:
(c) Since, its carbocation is stabilised by resonance as well as +1 effect.
9. CH3CH2CH2Br + NaCN → CH3CH2CH2CN + NaBr, will be fastest in
(a) ethanol
(b) methanol
(c) N, N dimethyl formamide
(d) Water
Answer/Explanation
Answer: c
Explaination: (c) It is favoured by non-polar solvent.
10.

Answer/Explanation
Answer: d
Explaination:
(d) It is substitution reaction but follows benzyne mechanism.
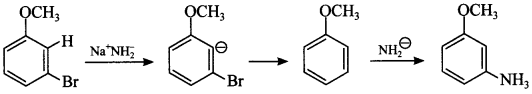
11. A dihalogen derivative ‘X’ of a hydrocarbon with three carbon atoms react with ale. KOH and produces hydrocarbon which forms red ppt. with ammonical Cu2Cl2. ‘X’ gives an aldehyde on reaction with aq. KOH. The compound ‘X’ is
(a) 1, 3-Dichloropropane
(b) 1, 2-Dichloropropane
(c) 2, 2-Dichloropropane
(d) 1, 1-Dichloropropane
Answer/Explanation
Answer: d
Explaination: d

12. The synthesis of alkyl fluoride is best accomplished by
(a) Finkelstein reaction
(b) Swartz reaction
(c) Free radical fluorination
(d) Sandmeyers reaction
Answer/Explanation
Answer: b
Explaination:
(b) C2H5Cl + AgF → C2H5F + AgCl.
13. How many chiral compounds are possible on monochlorination of 2-methyl butane?
(a) 2
(b) 4
(c) 6
(d) 8
Answer/Explanation
Answer:
Explaination:

14. The increasing order of reactivity towards SN1 mechanism is

(a) III < II < I
(b) II < I < III
(c) I < III < II
(d) II < III < I
Answer/Explanation
Answer: b
Explaination:
(b) ∵ 1° < 2° < Benzyl carbocation with OCH3 at p-position is order of stability of carbocation.
15. Arrange the following compounds in the increasing order of their densities. [NCERT Exemplar]

(a) (i) < (ii) < (iii) < (iv)
(b) (i) < (iii) < (iv) < (ii)
(c) (iv) < (iii) < (ii) < (i)
(d) (ii) < (iv) < (iii) < (i)
Answer/Explanation
Answer: a
Explaination: (a) Higher the molecular weight, higher will be density.
16. What is ‘A’ in the following reaction? [NCERT Exemplar]


Answer/Explanation
Answer: c
Explaination:
(c) It is MarkownikofFs addition, secondary carbocation is more stable.
17. Which of the following alkyl halides will undergo SN1 reaction most readily? [NCERT Exemplar]
(a) (CH3)3 C—F
(b) (CH3)3 C—Cl
(c) (CH3)3 C—Br
(d) (CH3)3 C—I
Answer/Explanation
Answer: d
Explaination:
(d) It is because C—I bond is weakest due to longer bond length.
18. Which of the carbon atoms present in the molecule given below are asymmetric? [NCERT Exemplar]

(a) 1, 2, 3, 4
(b) 2, 3
(c) 1, 4
(d) 1, 2, 3
Answer/Explanation
Answer: b
Explaination:
(b) 2 and 3 are chiral as these are linked with four different groups.
19. Which of the following compounds will give racemic mixture on nucleophilic substitution by OH” ion? [NCERT Exemplar]

Answer/Explanation
Answer: a
Explaination:
(a) Since it is optically active 2° halide, will follow SN1 mechanism.
20. Which of the following compounds will give racemic mixture on nucleophilic substitution by OH” ion? [NCERT Exemplar]
1-Bromoethane, 1-Bromopropane, 1-Bromobutane, Bromobenzene
(a) Bromobenzene < 1-Bromobutane < 1-Bromopropane < 1-Bromoethane
(b) Bromobenzene < 1-Bromoethane < 1-Bromopropane < 1-Bromobutane
(c) 1-Bromopropane < 1-Bromobutane < 1-Bromoethane < Bromobenzene
(d) 1-Bromoethane < 1-Bromopropane < 1-Bromobutane < Bromobenzene
Answer/Explanation
Answer: d
Explaination:
(d) Higher the molecular weight, higher will be boiling point.
21. SN1 reaction of alkyl halides lead to
(a) Retention of configuration
(b) Racemisation
(c) Inversion of configuration
(d) None of these
Answer
Answer: b
22. p-djchlorobenzene has higher melting point than its o- and m- isomers because
(a) p-dichlorobenzene is more polar than o- and m- isomer.
(b) p-isomer has a symmetrical crystalline structure.
(c) boiling point of p-isomer is more than o- and m-isomer.
(d) All of these are correct reasons.
Answer
Answer: b
23. Chloropicrin is formed by the reaction of
(a) steam on carbon tetrachloride.
(b) nitric acid on chlorobenzene.
(c) chlorine on picric acid.
(d) nitric acid on chloroform.
Answer
Answer: d
24. Fitting reaction can be used to prepare
(a) Toluene
(b) Acetophenon
(c) Diphenyl
(d) Chlorobenzene
Answer
Answer: c
25. Identify the end product (C) in the following sequence:

Answer
Answer: c
26.

In the above reaction, the product D is
(a) Propane
(b) 2, 3-Dimethylbutane
(c) Hexane
(d) Allyl bromide
Answer
Answer: b
27. Identify X and Y in the following sequence
![]()
(a) X = KCN, Y = LiAlH4
(b) X = KCN, Y = H3O+
(c) X = CH3Cl, Y = AlCl3 HCl
(d) X = CH3NH2, Y = HNO2
Answer
Answer: a
28. In the following sequence of reactions:
![]()
(a) n-propylamine
(b) isopropylamine
(c) ethylamine
(d) ethylmethylamine
Answer
Answer: d
29.

Answer
Answer: a
30.
Identifay Z in the series
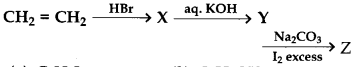
(a) C2H5I
(b) C2H5OH
(c) CHI3
(d) CH3CHO
Answer
Answer: c
Note: In the following questions two or more options may be correct. (Q.21 to Q.27)
Consider the following reaction and answer the questions No. 21-23.

31. Which of the statements are correct about above reaction? [NCERT Exemplar]
(a) (i) and (v) both are nucleophiles.
(b) In (iii) carbon atom is sp3 hybridised.
(c) In (iii) carbon atom is sp2 hybridised.
(d) (i) and (v) both are electrophiles.
Answer/Explanation
Answer:
Explaination:
(a) and (c) are correct.
(i) and (v) are -vely charged
∴ nucleophile. ‘C’ is sp² hybridised as C—OH and C—Cl bonds are half broken and half formed.
32. Which of the following statements are correct about this reaction? [NCERT Exemplar]
(a) The given reaction follows SN2 mechanism.
(b) (ii) and (iv) have opposite configuration.
(c) (ii) and (iv) have same configuration.
(d) The given reaction follows SN1 mechanism.
Answer/Explanation
Answer:
Explaination:
(a) and (b). In SN2 mechanism, inversion of configuration takes place due to back side attack.
33. Which of the following statements are correct about the reaction intermediate? [NCERT Exemplar]
(a) Intermediate (iii) is unstable because in this carbon is attached to 5 atoms.
(b) Intermediate (iii) is unstable because carbon atom is sp² hybridised.
(c) Intermediate (iii) is stable because carbon atom is sp² hybridised.
(d) Intermediate (iii) is less stable than the reactant (ii).
Answer/Explanation
Answer:
Explaination:
(a) and (d), intermediate is unstable than reactant as well as product as ‘C’ is bonded to 5 carbon atoms.
Answer Q. No. 24 and 25 on the basis of the following reaction.
(i) (ii) (iii) (iv)

34. Which of the following statements are correct about the mechanism of this reaction? [NCERT Exemplar]
(a) A carbocation will be formed as an intermediate in the reaction.
(b) OH– will attach the substrate (ii) from one side and Cl– will leave it simultaneously from other side.
(c) An unstable intermediate will be formed in which OH– and Cl– will be attached by weak bonds.
(d) Reaction proceeds through SN1 mechanism.
Answer/Explanation
Answer:
Explaination:
(a) and (d) are correct, In SN1 carbocation is formed. OH– can attract freely from either of the side.
35. Which of the following statements are correct about the kinetics of this reaction? [NCERT Exemplar]
(a) The rate of reaction depends on the concentration of only (ii).
(b) The rate of reaction depends on concentration of both (i) and (ii).
(c) Molecularity of reaction is one.
(d) Molecularity of reaction is two.
Answer/Explanation
Answer:
Explaination:
(a) and (d).
∴ Molecularity is 2 and it follows SN1 mechanism, therefore, rate depends upon cone, of 3° halide only.
36. Haloalkanes contain halogen atom (s) attached to the sp3 hybridised carbon atom of an alkyl group. Identify haloalkane from the following compounds. [NCERT Exemplar]
(a) 2-Bromopentane
(b) Vinyl chloride (chloroethene)
(c) 2-chloroacetophenone
(d) Trichloromethane
Answer/Explanation
Answer:
Explaination:
(a) 2-Bromopentane (d) Trichloromethane have sp3 hybridised ‘C’.
37. Ethylene chlonde and ethylidene chloride are isomers. Identify the correct statements. [NCERT Exemplar]
(a) Both the compounds form same product on treatment with alcoholic KOH.
(b) Both the compounds form same product on treatment with aq.NaOH.
(c) Both the compounds form same product on reduction.
(d) Both the compounds are optically active.
Answer/Explanation
Answer:
Explaination:

38. Match the the compounds given in Column I with the effects given in Column II. [NCERT Exemplar]
| Column I | Column II |
| (a) Chloramphenicol | (i) Malaria |
| (b) Thyroxine | (ii) Anaesthetic |
| (c) Chloroquine | (iii) Typhoid fever |
| (d) Chloroform | (iv) Goiter |
| (v) Blood substituent |
Answer/Explanation
Answer:
Explaination:
(a) (iii)
(b) (iv)
(c) (i)
(d) (ii)
39. Match the items of Column I and Column II. [NCERT Exemplar]
| Column I | Column II |
| (a) SN1 reaction | (i) vio-dibromides |
| (b) Chemicals in fire extinguisher | (ii) gem-dihalides |
| (c) Bromination of alkenes | (iii) Racemisation |
| (d) Alkylidene halides | (iv) Saytzeffrule |
| (e) Elimination of HX from alkylhalide | (v) Chlorobromocarbons |
Answer/Explanation
Answer:
Explaination:
(a) (iii)
(b) (v)
(c)(i)
(d) (ii)
(e) (iv)
40. Match the reactions given in Column I with the types of reactions given in Column II. [NCERT Exemplar]


Answer/Explanation
Answer:
Explaination:
(a) Aromatic electrophilic substitution (ii)
(b) Electrophilic addition (iv)
(c) Nucleophilic substitution (v)
(d) Nucleophilic aromatic substitution (i)
(e) Saytzeff elimination (iii)
Note: In the following questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices. (Q.31 to Q.35)
(a) Assertion and reason both are correct and reason is correct explanation of assertion.
(b) Assertion and reason both are wrong statements.
(c) Assertion is correct but reason is wrong statement.
(d) Assertion is wrong but reason is correct statement.
(e) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
41. Assertion: KCN reacts with methyl chloride to give methyl isocyanide.
Reason: CN– is an ambident nucleophile. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(d) Assertion is wrong but reason is correct statement. AgCN reacts with CH3Cl to give isocyanide, KCN gives cyanide. CN– is ambident nucleophile.
42. Assertion: fert-Butyl bromide undergoes Wurtz reaction to give 2, 2, 3, 3-tetramethylbutane.
Reason: In Wurtz reaction, alkyl halides react with sodium in dry ether to give hydrocarbon containing double the number of carbon atoms present in the halide. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(a) Assertion and reason both are correct and reason is correct explanation of assertion.

43. Assertion: Presence of a nitro group at ortho or para position increases the reactivity of haloarenes towards nucleophilic substitution.
Reason: Nitro group, being an electron withdrawing group decreases the electron density over the benzene ring. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(a) Assertion and reason both are correct and reason is correct explanation of assertion.
44. Assertion: In monohaloarenes, further electrophilic substitution occurs at ortho and para positions.
Reason: Halogen atom is a ring deactivator. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(e) Assertion and reason both are correct statements but reason is not correct explanation of assertion.
45. Assertion: Aryl iodides can be prepared by reaction of arenes with iodine in the presence of an oxidising agent.
Reason: Oxidising agent oxidises I2 into HI. [NCERT Exemplar]
Answer/Explanation
Answer:
Explaination:
(c) Assertion is correct but reason is wrong statement.
46. Chloromethane on treatment with excess of ammonia gives __________ .
Answer/Explanation
Answer:
Explaination: Methanamine
47. The isomer of C4H9Br, (optical active) is __________ .
Answer/Explanation
Answer:
Explaination:

48. Out of chlorobenzene, o-chlorotoluene, m-chloro toluene, least reactive towards nucleophilic substitution is __________ .
Answer/Explanation
Answer:
Explaination:
o-Chlorotoluene, because—CH3 group is electron releasing, decreases reactivity towards nucleophilic substitution reactions.
49.

is allylic halide. [True or False]
Answer/Explanation
Answer:
Explaination: True.
50. When benzene reacts with Cl2 and FeCl3, the attacking electrophile is Cl+. [True or False]
Answer/Explanation
Answer:
Explaination:
![]()
51. IUPAC name of Diethyl bromomethane is 3-Bromo-pentane.
Answer/Explanation
Answer:
Explaination: True.
52. Among isobutyl bromide, n-Butyl bromide, secondary butyl bromide and tertiary butyl bromide,
n-Butyl bromide has lowest boiling point. [True or False]
Answer/Explanation
Answer:
Explaination: False, it has highest boiling point 3° < isobutyl bromide < 2° < 1°.
53. Reactivity order of HI > HBr > HCl > HBr towards nucleophilic substitution reaction. [True or False]
Answer/Explanation
Answer:
Explaination: True. HI has lowest bond dissociation enthalphy.
54. Tert. halides undergo elimination reaction faster than nucleophilic substitution reaction. [True or False]
Answer/Explanation
Answer:
Explaination: True. Tertiary halides form carbocation followed by elimination.
55. Write the IUPAC name of the following compound:
CH2 =CHCH2Br [AI2011]
Answer/Explanation
Answer:
Explaination: 3-Bromoprop-1-ene.
56. Give the IUPAC name of the following compound. [Delhi 2012]

Answer/Explanation
Answer:
Explaination: 3-Bromo-2-methylprop-1 -ene.
57. Write the IUPAC name of the following compound: (CH3)3CCH2Br [Delhi 2011]
Answer/Explanation
Answer:
Explaination:

58. Write the IUPAC name of the following compound:
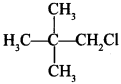
Answer/Explanation
Answer:
Explaination: 1-Chloro-2, 2-dimethylpropane.
59. Write IUPAC name of the given compound: [Delhi 2019]

Answer/Explanation
Answer:
Explaination: 4-chloro benzene sulphonic acid.
60. Draw the structure of 2-Bromopentane [Delhi 2014(C)]
Answer/Explanation
Answer:
Explaination:

61. Write the structure of the following compound:
2 -(2-chlorophenyl)-l-iodoethane. [AI 2011(C)]
Answer/Explanation
Answer:
Explaination:

62. How can you obtain iodoethane from ethanol when no other iodine containing reagent except Nal is available in the laboratory?
Answer/Explanation
Answer:
Explaination:

63. What happens when bromine attacks
![]()
Answer/Explanation
Answer:
Explaination:

64. Identify the products A and B formed in the following reaction:
![]()
Answer/Explanation
Answer:
Explaination:

65. How will you convert methyl iodide from methyl bromide? What is the name of this reaction?
Answer/Explanation
Answer:
Explaination:
This is Finkelstein reaction.

66. Arrange the following haloalkanes in the increasing order of their densities:
![]()
Answer/Explanation
Answer:
Explaination:
n-C3H7Cl < n-C3H7Br < n-C3H7I (Increasing order of density)
67. Which would undergo SN2 reaction faster in the following pair and why? [Delhi 2015]

Answer/Explanation
Answer:
Explaination:
CH3CH2Br will react faster due to less stearic hindrance.
68. Which would undergo SN1 reaction faster in the following pair:

Answer/Explanation
Answer:
Explaination:

will react faster due to 2°halide, will give stable carbocation.
69. Why is f-butyl bromide more reactive towards SN1 reaction as compared to n-butyl bromide? [Uttarakhand 2019]
Answer/Explanation
Answer:
Explaination:
It is because tert. butyl carbocation is more stable than n-butyl carbocation.
70. Which would undergo SN2 reaction faster in the following pair and why? [Foreign 2015]
CH3—CH2—Cl or C6H6CH2Cl
Answer/Explanation
Answer:
Explaination:
CH3CH2l will undergo SN2 reaction faster because it has lower bond dissociation enthalpy of C—I bond due to longer bond length, r is better leaving group.
71. Which would undergo SN1 reaction faster in the following pair and why? [AI 2015]

Answer/Explanation
Answer:
Explaination:

because it forms stable 3° carbocation.
72. Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? [Uttarakhand 2019]

Answer/Explanation
Answer:
Explaination:

will react faster because it has less stearic hindrance.
73. Which will react faster in SN2 displacement, 1-bromopentane or 2-bromopentane, and why? [Foreign 2011]
Answer/Explanation
Answer:
Explaination:
1-Bromopentane will react faster in SN2 because it is 1° (primary) halide and has less stearic hindrance.
74. A solution of KOH hydrolyses CH3CHClCH2CH3 and CH3CH2CH2CH2Cl. Which one of these is more easily hydrolysed?
Answer/Explanation
Answer:
Explaination:
CH3—CH(Cl)CH2CH3 will be more easily hydrolysed because it will form secondary carbocation which is more stable than primary carbocation.
75. Which of the compounds will react faster in SN1 reaction with the OH– ion?
CH3—CH2—Cl or C6H5—CH2—Cl
Answer/Explanation
Answer:
Explaination:
C6H5CH2Cl will react faster due to formation of resonance stabilised carbocation.
76. Explain why the following pairs of compound do not show optical activity.

Answer/Explanation
Answer:
Explaination:
It is racemic mixture which contains equal amounts of dextro and laevorotatory substances. If half of the total number of molecules rotates the plane-polarised light towards left, remaining half of the molecules rotates towards right such that net optical rotation is zero.
77. Identify the chiral molecule in the following pair: [AI 2014]

Answer/Explanation
Answer:
Explaination:
![]()
78. What is meant by enantiomers?
Answer/Explanation
Answer:
Explaination:
The stereoisomers which are non-superimposable mirror images are called enantiomers, e.g. d(+) glucose and l(-) glucose are enantiomers.
79. What is the major product formed from dehydrohalogenation of 2-Bromopentane?
Answer/Explanation
Answer:
Explaination:
Pen-2-ene (81%)

80. Give the chemical reaction involved in the formation of Grignard reagent. [Foreign 2011]
Answer/Explanation
Answer:
Explaination:

We hope the given Chemistry MCQs for Class 12 with Answers Chapter 10 Haloalkanes and Haloarenes will help you. If you have any query regarding CBSE Class 12 Chemistry Haloalkanes and Haloarenes MCQs Pdf, drop a comment below and we will get back to you at the earliest.