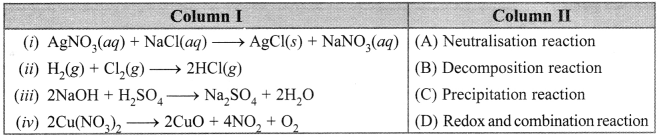
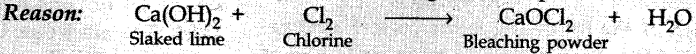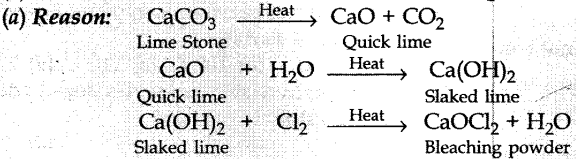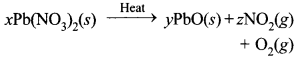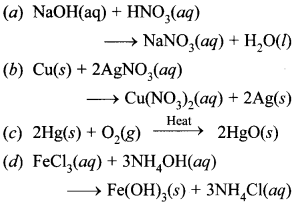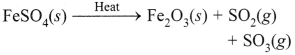Free PDF Download of CBSE Class 10 Science Chapter 2 Acids Bases and Salts Multiple Choice Questions with Answers. MCQ Questions for Class 10 Science with Answers was Prepared Based on Latest Exam Pattern. Students can solve NCERT Class 10 Science Acids Bases and Salts Multiple Choice Questions with Answers to know their preparation level.
Class 10 Science MCQs Chapter 2 Acids Bases and Salts
1. What happens when a solution of an acid is mixed with a solution of a base in a test tube?
(i) Temperature of the solution decreases
(ii) Temperature of the solution increases
(in) Temperature of the solution remains the same
(iv) Salt formation takes place
(a) (i) and (iv)
(b) (i) and (iii)
(c) (ii) only
(d) (ii) and (iv)
Answer
Answer: d
2. When hydrogen chloride gas is prepared on a humid day, the gas is usually passed through the guard tube containing calcium chloride. The role of calcium chloride taken in the guard tube is to
(a) absorb the evolved gas
(b) moisten the gas
(c) absorb moisture from the gas
(d) absorb Cl– ions from the evolved gas
Answer/ Explanation
Answer: c
Explaination: Reason: Guard tube drys (absorbs water) from calcium chloride on a humid day.
3. Which one of the following salts does not con-tain water of crystallisation?
(a) Blue vitriol
(b) Baking soda
(c) Washing soda
(d) Gypsum
Answer
Answer: b
4. In terms of acidic strength, which one of the following is in the correct increasing order?
(a) Water < Acetic acid < Hydrochloric acid
(b) Water < Hydrochloric acid < Acetic acid
(c) Acetic acid < Water < Hydrochloric acid
(d) Hydrochloric acid < Water < Acetic acid
Answer
Answer: a
5. What is formed when zinc reacts with sodium hydroxide?
(a) Zinc hydroxide and sodium
(b) Sodium zincate and hydrogen gas
(c) Sodium zinc-oxide and hydrogen gas
(d) Sodium zincate and water
Answer/ Explanation
Answer: b
Explaination: Reason: Zn + 2NaOH → Ma2Zn02 (Sodium Zincate) + H2
6. Tomato is a natural source of which acid?
(a) Acetic acid
(b) Citric acid
(c) Tartaric acid
(d) Oxalic acid
Answer
Answer: d
7. Brine is an
(a) aqueous solution of sodium hydroxide
(b) aqueous solution of sodium carbonate
(c) aqueous solution of sodium chloride
(d) aqueous solution of sodium bicarbonate
Answer
Answer: c
8. Na2CO3 . 10H2O is
(a) washing soda
(b) baking soda
(c) bleaching powder
(d) tartaric acid
Answer
Answer: a
9. At what temperature is gypsum heated to form Plaster of Paris?
(a) 90°C
(b) 100°C
(c) 110°C
(d) 120°C
Answer
Answer: b
10. How many water molecules does hydrated cal-cium sulphate contain?
(a) 5
(b) 10
(c) 7
(d) 2
Answer/ Explanation
Answer: d
Explaination: Reason: Chemical formula of hydrated calcium sulphate or gypsum is CaSO4.2H2O
11. Sodium carbonate is a basic salt because it is a salt of a
(a) strong acid and strong base
(b) weak acid and weak base
(c) strong acid and weak base
(d) weak acid and strong base
Answer
Answer: d
12. Alkalis are
(a) acids, which are soluble in water
(b) acids, which are insoluble in water
(c) bases, which are insoluble in water
(d) bases, which are soluble in water
Answer
Answer: d
13. Which of the following statements is correct about an aqueous solution of an acid and of a base?
(i) Higher the pH, stronger the acid
(ii) Higher the pH, weaker the acid
(in) Lower the pH, stronger the base
(iv) Lower the pH, weaker the base
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer/ Explanation
Answer: d
Explaination: Reason: Stronger the acid, lesser is the pH. Stronger the base, higher is the pH.
14. The apparatus given in the adjoining figure was set up to demonstrate electrical conductivity.

Which of the following statement(s) is (are) correct?
(i) Bulb will not glow because electrolyte is not acidic.
(ii) Bulb will glow because HCl is a strong acid and furnishes ions for conduction.
(iii) Bulb will not glow because circuit is incomplete.
(iv) Bulb will not glow because it depends upon the type of electrolytic solution.
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (ii) only
(d) (iv) only
Answer
Answer: c
15. Lime water reacts with chlorine to give
(a) bleaching powder
(b) baking powder
(c) baking soda
(d) washing soda
Answer/ Explanation
Answer: c
Explaination:
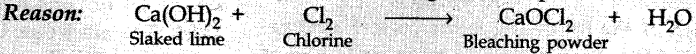
16. Nettle sting is a natural source of which acid?
(a) MetiWanoic acid
(b) Lactic acid
(c) Citric acid
(d) Tartaric acid
Answer
Answer: a
17. Tooth enamel is made up of
(a) calcium phosphate
(b) calcium carbonate
(c) calcium oxide
(d) potassium
Answer
Answer: a
18. What is the pH range of our body?
(a) 7.0 – 7.8
(b) 7.2 – 8.0
(c) 7.0 – 8.4
(d) 7.2 – 8.4
Answer
Answer: a
19. Rain is called acid rain when its:
(a) pH falls below 7
(b) pH falls below 6
(c) pH falls below 5.6
(d) pH is above 7
Answer
Answer: c
20. Sodium hydroxide is a
(a) weak base
(b) weak acid
(c) strong base
(d) strong acid
Answer/ Explanation
Answer: c
Explaination: Reason: Sodium hydroxide ionises in water and produces a large amount of hydroxide ions.
21. An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change?
(a) Baking powder
(b) Lime
(c) Ammonium hydroxide solution
(d) Hydrochloric acid
Answer
Answer: d
22. When copper oxide and dilute hydrochloric acid react, colour changes to
(a) white
(b) bluish-green
(c) blue-black
(d) black
Answer/ Explanation
Answer: b
Explaination: Reason: Blue-green colour of solution is due to the formation of copper (II) chloride.
23. Sodium hydroxide is used
(a) as an antacid
(b) in manufacture of soap
(c) as a cleansing agent
(d) in alkaline batteries
Answer
Answer: b
24. Sodium hydroxide turns phenolphthalein solution
(a) pink
(b) yellow
(c) colourless
(d) orange
Answer
Answer: a
25. Chemical formula of washing soda is
(a) Na2C03 . 7H2O
(b) Na2C03 . 5H2O
(c) Na2C03 . 2H2O
(d) Na2C03 . 10H2O
Answer
Answer: d
26. Which of the following is not a acidic salt?
(a) CuSO4
(b) NH4Cl
(c) FeCl3
(d) CH3COONa
Answer
Answer: d
27. A solution of NaCl
(i) will turn red litmus blue
(ii) will turn pH paper green
(iii) will turn blue litmus red
(iv) will not affect litmus
(a) (i) and (ii)
(b) (i), and, (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer
Answer: d
28. Many salts absorbs water from atmosphere. This property is called
(a) deliquescence
(b) efflorescence
(c) hydration
(d) addition
Answer
Answer: a
29. An aqueous solution with pH = 1 is
(a) strongly acidic
(b) strongly basic
(c) neutral
(d) weakly acidic
Answer
Answer: a
30. CaOCl2 will liberate Cl2 gas in presence of
(i) CO2
(ii) HCl
(iii) CO
(iv) NO
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer
Answer: a
31. Egg shell is made up of
(a) CaCO3
(b) CaO
(c) Ca(OH)2
(d) CaCl2
Answer
Answer: a
32. Curd cannot be stored in
(z) Brass vessel
(ii) Copper vessel
(iii) Steel
(iv) Bronze
(a) (i), (ii), (iii)
(b) (ii), (iii), (iv)
(c) (i), (ii), (iv)
(d) (i), (iii), (iv)
Answer
Answer: c
33. What happens when a solution of an acid is mixed with a solution of a base in a test tube? [NCERT Exemplar Problems]
(i) The temperature of the solution increases
(ii) The temperature of the solution decreases
(iii) The temperature of the solution remains the same
(iv) Salt formation takes place.
(a) (i) only
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (i) and (iv)
Answer
Answer: d
34. An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change? [NCERT Exemplar Problems]
(a) Baking power
(b) Lime
(c) Ammonium hydroxide solution
(d) Hydrochloric acid
Answer
Answer: d
35. During the preparation of hydrogen chloride gas on a humid day, the gas is usually passed through the guard tube containing calcium chloride. The role of calcium chloride taken in the guard tube is to [NCERT Exemplar Problems]
(a) absorb the evolved gas
(b) moisten the gas
(c) absorb moisture from the gas
(d) absorb CL ions from the evolved gas
Answer
Answer: c
36. Which of the following salts does not contain water of crystallisation? [NCERT Exemplar Problems]
(a) Blue vitriol
(b) Baking soda
(c) Washing soda
(d) Gypsum
Answer
Answer: b
37. Sodium carbonate is a basic salt because it is a salt of [NCERT Exemplar Problems]
(a) strong acid and strong base.
(b) weak acid and weak base.
(c) strong acid and weak base.
(d) weak acid and strong base.
Answer
Answer: d
38. Calcium phosphate is present in tooth enamel. Its nature is [NCERT Exemplar Problems]
(a) basic
(b) acidic
(c) neutral
(d) amphoteric
Answer
Answer: a
39. A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange. Which of the following would change the colour of this pH paper to greenish-blue? [NCERT Exemplar Problems]
(a) Lemon juice
(b) Vinegar
(c) Common salt
(d) An antacid
Answer
Answer: d
40. Which of the following gives the correct increasing order of acidic strength ? [NCERT Exemplar Problems]
(a) Water < Acetic acid < Hydrochloric acid
(b) Water < Hydrochloric acid < Acetic acid
(c) Acetic acid < Water < Hydrochloric acid
(d) Hydrochloric acid < Water < Acetic acid
Answer
Answer: a
41. Which of the following phenomena occur, when a small amount of acid is added to water ?
Answer
Answer: b
42. Which one of the following can be used as an acid-base indicator by a visually impared (blind) student ?
(a) Litmus
(b) Turmeric
(c) Vanilla essence
(d) Petunia leaves
Answer
Answer: c
43. In an attempt to demonstrate electrical conductivity through an electrolyte, the following apparatus was set up.

Which among the following statement)s) is (are) correct?
(i) Bulb will not glow because electrolyte is not acidic.
(ii) Bulb will glow because NaOH is a strong base and furnishes ions for conduction.
(iii) Bulb will not glow because circuit is incomplete.
(iv) Bulb will not glow because it depends upon the type of electrolytic solution.
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (ii) only
(d) (iv) only
Answer
Answer: c
44. Which of the following is used for dissolution of gold ? [NCERT Exemplar Problems]
(a) Hydrochloric acid
(b) Sulphuric acid
(c) Nitric acid
(d) Aqua regia
Answer
Answer: d
45. Which among the following is not a base? [NCERT Exemplar Problems]
(a) NaOH
(b) KOH
(c) NH4OH
(d) C2H5OH
Answer
Answer: d
46. Which of the following statement is not correct? [NCERT Exemplar Problems]
(a) All metal carbonates react with acid to give salt, water and carbon dioxide.
(b) All metal oxides react with water to give salt and acid.
(c) Some metals react with acids to give salt and hydrogen.
(d) Some non-metal oxides react with water to form an acid.
Answer
Answer: b
47. Equal volumes of hydrochloric acid and sodium hydroxide solutions of same concentration are mixed and the pH of the resulting solution is checked with a pH paper. What would be the colour obtained? (You may use colour guide given in figure)
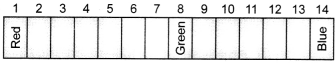
(a) Red
(b) Yellow
(c) Yellowish green
(d) Blue
Answer
Answer: c
48. Which of the following is(are) true when HCl (g) is passed through water ? [NCERT Exemplar Problems]
(i) It does not ionise in the solution as it is a covalent compound.
(ii) It ionises in the solution.
(iii) It gives both hydrogen and hydroxyl ion in the solution.
(iv) It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule.
(a) (i) only
(b) (iii) only
(c) (ii) and (iv)
(d) (iii) and (iv)
Answer
Answer: c
49. Which of the following statements is true for acids? [NCERT Exemplar Problems]
(a) Bitter and change red litmus to blue.
(b) Sour and change red litmus to blue.
(c) Sour and change blue litmus to red.
(d) Bitter and change blue litmus to red.
Answer
Answer: c
50. Which of the following are present in a dilute aqueous solution of hydrochloric acid? [NCERT Exemplar Problems]
(a) H3O+ + Cl–
(b) H3O+ + OH–
(c) Cl– + OH–
(d) unionised HCl
Answer
Answer: a
51. NaHCO, formed by reaction of
(a) NaOH + H2CO3
(b) NaCl + H2CO3
(c) Na2CO3 + HCl
(d) NaOH + Na2CO3
Answer/Explanation
Answer: a
Explanation:
(a) NaOH + H2CO3 → NaHCO3 + H2O
52. pH of H20 is
(a) 7
(b) 8
(c) 9
(d) 10
Answer/Explanation
Answer: a
Explanation:
(a) pH of H2O is 7 because it is neutral.
53. Ag2S reacts with H2SO4 to form
(a) AgSO4
(b) Ag2SO4 + H2S
(c)Ag2O + H2S
(d) AgOH + H2S
Answer/Explanation
Answer: b
Explanation:
(b) Ag2S + H2SO4 → Ag2SO4 + H2S
54. Lime water reacts with chlorine to form
(a) CaCl2
(b) CaOCl2
(c) Ca(ClO3)2
(d) CaO2Cl2
Answer/Explanation
Answer: b
Explanation:
(b) Ca(OH)2 + Cl2 -> CaOCl2 + H2O
55. NaOH is obtained by electrolysis of
(a) Aq. solution of NaCl
(b) Aq. Na2CO3
(c) Aq. NaHCO3
(d) Molten NaCl
Answer/Explanation
Answer: a
Explanation:
(a) 2NaCl + 2H2O → 2NaOH + H2 + Cl2
Direction (Q56 to Q60): In the following Questions, the Assertion and Reason have been put forward. Read the statements carefully and choose the correct alternative from the following:
(a) Both the Assertion and the Reason are correct and the Reason is the correct explanation of the Assertion.
(b) The Assertion and the Reason are correct but the Reason is not the correct explanation of the Assertion.
(c) Assertion is true but the Reason is false.
(d) The statement of the Assertion is false but the Reason is true.
56. Assertion: Carbonic acid is weak acid
Reason: It ionised completely in aqueous solution.
Answer/Explanation
Answer: c
Explanation:
(c) Assertion is true but the Reason is false.
57. Assertion: Copper sulphate is acidic salt.
Reason: It is a salt of weak base [Cu(OH)7] and strong acid (H7SO4).
Answer/Explanation
Answer: a
Explanation:
(a) Both the Assertion and the Reason are correct and the Reason is the correct explanationof the Assertion.
58. Assertion: Ammonium hydroxide is Weak Base
Reason: Phenolphthalein becomes pink in NH2OH
Answer/Explanation
Answer: b
Explanation:
(b) The Assertion and the Reason are correct but the Reason is not the correct explanationof the Assertion.
59. Assertion: Bleaching power liberate chlorine when kept in atmosphere.
Reason: CaOCl2 reacts with CO2 present in atmosphere to form CaCO3 and chlorine gas.
Answer/Explanation
Answer: a
Explanation:
(a) Both the Assertion and the Reason are correct and the Reason is the correct explanationof the Assertion.
60. Assertion: Universal indicator gives green colour with distilled water.
Reason: pH of distilled water is 7 and it is neutral and universal indicator gives green colour with neutral solution.
Answer/Explanation
Answer: a
Explanation:
(a) Both the Assertion and the Reason are correct and the Reason is the correct explanationof the Assertion.
61. One of the constituents of baking powder is sodium hydrogen carbonate, the other constituent is _____________ .
Answer/Explanation
Answer: a
Explanation: tartaric acid
62. The three most common indicators to test for acid and bases are litmus _____________ and _____________ .
Answer/Explanation
Answer: a
Explanation: methyl orange, phenolphthalein
63. Acid reacts with metal to form salt and _____________ gas.
Answer/Explanation
Answer: a
Explanation: hydrogen
64. The aqueous solution of an acid or a base conducts electricity due to the presence of _____________ in it.
Answer/Explanation
Answer: a
Explanation: ions
65. A base which is soluble in water is known as an _____________ .
Answer/Explanation
Answer: a
Explanation: alkali
66. The salts of carbonic acids are called _____________ .
Answer/Explanation
Answer: a
Explanation: carbonates
67. Methyl orange indicator gives red colour in acidic solution and yellow colour in basic solution. [True/False]
Answer/Explanation
Answer: a
Explanation: True
68. All the organic acids are strong acids. [True/False]
Answer/Explanation
Answer: a
Explanation: False
69. The dilution of a concentrated acid should always be done by adding concentrated acid to water gradually with stirring. [True/False]
Answer/Explanation
Answer: a
Explanation: True
70. Glucose solution conduct electricity. [True/False]
Answer/Explanation
Answer: a
Explanation: False
71. The strength of an acid or base is measured on a scale of numbers called pH paper. [True/False]
Answer/Explanation
Answer: a
Explanation: False
72. The more alkaline a solution, the more is the pH. [True/False]
Answer/Explanation
Answer: a
Explanation: True
Direction (Q48 to Q49): Match Column I with Column II.
73.
| Column I |
Column II |
| A. Bleaching powder |
(i) Preparation of glass |
| B. Plaster of Paris |
(ii) Production of H2 and Cl2 |
| C. Washing soda |
(iii) Manufacture of chalk |
| D. Baking soda |
(iv) Antacid |
| E. Sodium chloride |
(v) Decolourisation |
Answer/Explanation
Answer: a
Explanation:
A (v)
B (iii)
C (i)
D (iv)
E (ii)
74.
| Column I |
Column II |
| A. Plaster of Paris |
(i) CaSO4 . 2H2O |
| B. Bleaching powder |
(ii) CaO |
| C. Washing soda |
(iii) CaSO4 . 1/2H2O |
| D. Gypsum |
(iv) Na2CO3 . 10H2O |
| E. Slaked lime |
(v) Ca(OH) 2 |
| F. Quick lime |
(vi) CaOCl2 |
Answer/Explanation
Answer: a
Explanation:
A (iii)
B (vi)
C (iv)
D (i)
E (ii)
F (v)
75. What are indicators?
Answer/Explanation
Answer: a
Explanation:
Those substances which change their colour in different types of substances are called indicators.
76. What is litmus solution?
Answer/Explanation
Answer: a
Explanation:
It is purple dye which is extracted from a plant ‘lichen’. It is used as acid-base indicator.
77. Name a salt which does not contain water of crystallisation. [DoE]
Answer/Explanation
Answer: a
Explanation:
Salts like barium sulphate, potassium chloride, sodium nitrate, etc does not contain water of crystallisation.
78. Define universal indicator.
Answer/Explanation
Answer: a
Explanation:
A universal indicator is a mixture of indicators which shows a gradual but well marked series of colour changes over a very wide range of change in concentration of H+ ions.
79. Name an indicator which indicates the various levels of hydrogen ion concentration.
Answer/Explanation
Answer: a
Explanation: Universal indicator.
80. Name flowers whose colour or petals can be used as indicator.
Answer/Explanation
Answer: a
Explanation: Hydrangea, Petunia, Geranium
81. What are phenolphthalein and methyl orange?
Answer/Explanation
Answer: a
Explanation: Synthetic indicators
82. What is the colour of methyl orange in NaOH?
Answer/Explanation
Answer: a
Explanation: Yellow.
83. What is the colour of phenolphthalein in milk of magnesia?
Answer/Explanation
Answer: a
Explanation: Pink.
84. What is the colour of turmeric in soap solution?
Answer/Explanation
Answer: a
Explanation: Reddish-brown.
85. What will happen to red litmus solution when it is added to bitter gourd extract? Is it acidic or basic?
Answer/Explanation
Answer: a
Explanation: It changes to blue. It is basic in nature.
86. What will happen to blue litmus when it is added to soda water?
Answer/Explanation
Answer: a
Explanation: It changes to red.
87. Dry ammonia gas has no action on litmus paper, but a solution of ammonia in water turns red litmus paper blue. Why is it so?
Answer/Explanation
Answer: a
Explanation:
Ammonia dissolves in water, forms ammonium hydroxide which is base and turns red litmus blue.
NH3 + H2O → NH4+ + OH–
Dry ammonia gas does not change into OH– ions.
88. What would be the colour of litmus in a solution of sodium carbonate?
Answer/Explanation
Answer: a
Explanation:
The red litmus will change to blue in sodium carbonate solution.
89. Name an acid-base indicator prepared at home.
Answer/Explanation
Answer: a
Explanation:
Beetroot extract.
90. What is the colour of litmus in a solution of ammonium hydroxide?
Answer/Explanation
Answer: a
Explanation:
Red litmus will turn blue in ammonium hydroxide.
91. A bud of petunia became reddish purple after first shower of rain. What does it indicate?
Answer/Explanation
Answer: a
Explanation:
The rain was acid-rain.
92. How will you test for the gas which is liberated when hydrochloric acid reacts with an active metal? [CBSE 2012]
Answer/Explanation
Answer: a
Explanation:
Bring a burning matchstick near the gas. It bums with ‘pop’ sound showing that it is hydrogen.
93. Which gas is evolved when sodium hydroge-ncarbonate reacts with dilute hydrochloric acid?
Answer/Explanation
Answer: a
Explanation:
Carbon dioxide gas is evolved.
94. A few drops of sulphuric acid is added to water before electrolysis, why? [CBSE 2011]
Answer/Explanation
Answer: a
Explanation:
It makes water better conductor.
95. What effect does the concentration of H+(ag) have on the acidic nature of the solution?
Answer/Explanation
Answer: a
Explanation:
Acidic nature increases with increase in cone, of H3O+ ion.
96. Which planet contains yellowish clouds of sulphuric acid?
Answer/Explanation
Answer: a
Explanation: Venus.
97. Why does HCl(g) not conduct electricity when dissolved in toluene?
Answer/Explanation
Answer: a
Explanation:
It is because HCl cannot ionise in organic solvent like toluene.
98. How will you test a gas which is liberated when HCl acid reacts with an active metal? [DoE]
Answer/Explanation
Answer: a
Explanation:
The gas liberated is hydrogen gas. If we bring a burning splinter near the test tube, the splinter will extinguish with a pop sound.
99. Which gas is evolved when metal carbonates or metal hydrogen carbonate reacts with dilute acids?
Answer/Explanation
Answer: a
Explanation: CO2(g)
100. Which acid is present in honey bee sting and hair of nettle leaves?
Answer/Explanation
Answer: a
Explanation: Formic acid
Fill in the blanks
1. Acids turn …………. litmus solution…………. .
2. pH of basic solution is always …………. than 7.
3. …………. are the products obtained when bleaching powder reacts with dilute sulphuric acid.
4. Potassium nitrate has pH value equal to …………. .
5. …………. is the fixed number of water molecules chemically attached to each formula unit of a salt in its crystalline form.
6. …………. is one of the raw materials for the production of baking soda.
7. The salts of a strong acid and weak base are …………. with pH value …………. than 7.
8. Use of mild base like …………. on the bee-stung area gives relief.
9. During indigestion the stomach produces too much …………. and this causes pain and irritation.
10. The presence of …………. Ca in acids is responsible for their acidic properties.
11. Mixing an acid or base with water results in decrease in the concentration of per unit volume.
This process is called
12. Among HCl, H2SO4 and CH3COOH, …………. is a weak acid.
Answers
1. blue, red
2. more/greater
3. CaSO4, Cl2, H2O
4. 7 or seven
5. Water of crystallisation
6. Sodium chloride
7. acidic, less
8. baking soda
9. acid (HCl)
10. H+
11. OH– ions/H3O+ ions, dilution
12. CH3COOH
We hope the given MCQ Questions for Class 10 Science Acids Bases and Salts with Answers will help you. If you have any query regarding CBSE Class 10 Science Chapter 2 Acids Bases and Salts Multiple Choice Questions with Answers, drop a comment below and we will get back to you at the earliest.